Abstract
Purpose
Corticosteroids are now recommended for patients with severe COVID-19 including those with COVID-related ARDS. This has generated renewed interest regarding whether corticosteroids should be used in non-COVID ARDS as well. The objective of this study was to summarize all RCTs examining the use of corticosteroids in ARDS.
Methods
The protocol of this study was pre-registered on PROSPERO (CRD42020200659). We searched online databases including MEDLINE, EMBASE, CDC library of COVID research, CINAHL, and COCHRANE. We included RCTs that compared the effect of corticosteroids to placebo or usual care in adult patients with ARDS, including patients with COVID-19. Three reviewers abstracted data independently and in duplicate using a pre-specified standardized form. We assessed individual study risk of bias using the revised Cochrane ROB-2 tool and rated certainty in outcomes using GRADE methodology. We pooled data using a random effects model. The main outcome for this review was 28-day-mortality.
Results
We included 18 RCTs enrolling 2826 patients. The use of corticosteroids probably reduced mortality in patients with ARDS of any etiology (2740 patients in 16 trials, RR 0.82, 95% CI 0.72–0.95, ARR 8.0%, 95% CI 2.2–12.5%, moderate certainty). Patients who received a longer course of corticosteroids (over 7 days) had higher rates of survival compared to a shorter course.
Conclusion
The use of corticosteroids probably reduces mortality in patients with ARDS. This effect was consistent between patients with COVID-19 and non-COVID-19 ARDS, corticosteroid types, and dosage.
Similar content being viewed by others
Corticosteroids probably reduce mortality and duration of mechanical ventilation and these results were consistent in both COVID and non-COVID ARDS. Corticosteroids should likely be used in most patients with ARDS, regardless of etiology. |
Introduction
The role of corticosteroids in acute respiratory distress syndrome (ARDS) remains controversial [1]. Although there are a number of randomized control trials (RCTs) [2, 3] that have shown benefit of corticosteroids in ARDS, practice remains variable [1]. Also, observational data, although limited by confounding and imbalances in baseline characteristics, suggest that in certain subtypes of ARDS, such as viral ARDS caused by influenza, corticosteroids may be associated with increased mortality [4]. Furthermore, short-term steroid use has been associated with opportunistic infections, even in immunocompetent hosts [5, 6]. Ongoing questions related to optimal dosage, duration, initiation of treatment, and type of corticosteroids likely also contribute variation in use.
The emergence of data suggesting corticosteroids improve survival in severe coronavirus disease 2019 (COVID-19) has led to renewed interest in the overall effects of corticosteroids in ARDS [7]. Pooled results from recent RCTs in critically ill patients with COVID-19 show a reduction in mortality with the use of systemic corticosteroids (odds ratio [OR] 0.66, 95% confidence interval [CI] 0.53–0.82) [7]. These results informed a WHO clinical practice guideline which provided a strong recommendation for corticosteroids in patients with severe COVID-19 [8]. This new evidence has led experts to hypothesize that the results of these COVID studies may be generalizable to ARDS of non-COVID etiology [1]. Given that all ARDS is in part a result of a hyper-inflammatory response to a direct injury [9], data derived from COVID-19 studies may also inform the care of non-COVID ARDS patients.
The objective of this systematic review and meta-analysis was to summarize the RCT data examining the use of corticosteroids in ARDS of any cause. We hypothesized that corticosteroid administration would be beneficial in all patients with ARDS regardless of cause. In addition, we examined whether the effects of corticosteroids are consistent across COVID-19 and non-COVID 19 ARDS, corticosteroid dosing regimes, time of corticosteroid initiation, duration of therapy and amongst different types of corticosteroid molecules.
Methods
Protocol and registration
The protocol of this study was pre-registered on PROSPERO (CRD42020200659) and findings are reported using the PRISMA checklist (e-Table 1).
Search and information sources
Authors of this manuscript (MJM, PE, WA, BR, ZY, LCL, FL) have previously published three systematic reviews addressing a similar clinical question [4, 10, 11]. We made sure to incorporate all the eligible studies from these systematic reviews as part of our analysis and then proceeded to update the previous searches from February 15, 2020 to September 6, 2020.
For the update, we performed a comprehensive search of relevant databases (MEDLINE, EMBASE, Centre for Disease Control (CDC) library of COVID research, CINAHL and COCHRANE centre for trials). We limited our search to humans, but included any language. A copy of our search strategy can be found in our online Supplementary Materials. We screened reference lists of relevant systematic reviews and meta-analysis and also contacted experts in the field to ensure we were not missing any additional articles.
Study selection
We screened all citations in duplicate (DC, SS) in two stages. First, we screened titles and abstracts, and then for any citation selected in this first stage, we screened the full texts. We captured reasons for exclusion during full text review. A third reviewer (BR) adjudicated disagreements, when necessary.
Eligibility criteria
We included RCTs that compared corticosteroids to placebo or usual care in adult patients with ARDS (as defined by the American-European Consensus Conference (AECC) criteria [12] or the Berlin criteria for ARDS [13]). As some of the potentially eligible studies were not explicit about ARDS diagnosis, we made the decision to include studies of COVID-19 patients if they were receiving mechanical ventilation, as these were felt to most likely represent a population or subpopulation consistent with ARDS. We excluded case series, case studies, cohort studies, case reports and other observational studies. We focused on the following outcomes of interest: mortality (if multiple time points were provided, we chose the time point closest to 28 day mortality), duration of mechanical ventilation, intensive care unit (ICU) length of stay, hospital length of stay, incidence of opportunistic infections (as defined by the study authors), muscle weakness (as defined by the study authors), gastrointestinal bleeding and hyperglycemia (both as defined by study authors).
Data collection process and data items
Three reviewers (DC, AK, KS) abstracted data independently and in duplicate using a pre-specified standardized data abstraction form. A fourth reviewer (BR) adjudicated disagreements. We collected data on trial characteristics, demographic data, intervention and control procedures, and outcomes of interest. In the case of missing data, we contacted the study authors.
Risk of bias assessment in individual studies
We assessed risk of bias independently and in duplicate using the Cochrane Risk of Bias 2.0 tool for RCTs. We used the tool to assess for risk of bias (ROB) in the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. We rated each domain as “low”, “some concerns” or “high”. We determined overall ROB for each trial based on the highest risk attributed to any one domain. We assessed certainty of evidence for each outcome using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [14]. In keeping with GRADE methods, we use terminology consistent with the overall certainty of evidence. This includes stronger language for high certainty evidence, and less certain language (‘probably’ or ‘may’) for moderate or low certainty evidence.
Summary measures and synthesis of results
We used the DerSimonnian–Laird random effects model with inverse-variance weighting to generate pooled treatment effects across studies. We assessed heterogeneity between trials using a combination of the Chi2 test, the I2 statistic, and visual inspection of the forest plots. We present results of dichotomous outcomes using relative risk (RR) and continuous outcomes as mean difference (MD), both with 95% confidence intervals (CIs). We have also provided absolute differences with 95% CIs which we used for GRADE ratings. If medians and interquartile ranges (IQR) were reported instead of mean and standard deviation (SD), we assumed normality in data distribution and converted IQR to SDs by dividing the IQR by 1.35 [15].
Subgroup analysis and trial sequential analysis (TSA)
We considered a number of a priori subgroup analyses for the outcome of mortality, including: type of corticosteroid used (methylprednisolone vs dexamethasone vs hydrocortisone), timing of corticosteroid initiation after diagnosis of ARDS (early ≤ 72 h vs late corticosteroid initiation > 72 h), protocolize duration of corticosteroid therapy (≤ 7 vs > 7 days), corticosteroid dose (below median daily dose (< 88 mg/day of methylprednisolone equivalent) used in included trials vs above median daily dose used in included trials), ARDS etiology (COVID-19 ARDS vs. non COVID-19 ARDS), and ROB (high ROB studies vs low ROB). For ROB, all studies deemed at high ROB or having some concerns regarding ROB were analyzed as part of the high ROB subgroup, with the remainder being analyzed as part of the low ROB subgroup. We also performed three post-hoc subgroup analyses which were requested by peer reviewers: (1) studies published before 2015 as compared to those published on or after 2015, examining the impact in care evolution (such increased use of low tidal volume ventilation); (2) studies that had a placebo arm as compared to those that used standard or usual care and (3) studies that included patients who met formal ARDS criteria as per AECC or Berlin and studies that did not.
For all subgroup analysis, if the p value for the Chi-squared test was < 0.05, we used the ICEMAN tool [16] to assess for credible subgroup effects. This tool considers factors such as the following: whether effect modification is based on comparisons within rather than between trials; whether the effect modification was correctly hypothesized a priori; whether the effect modification was supported by prior evidence; how many subgroups were investigated; and whether random or fixed effects model were used. In all other cases, we assumed that the subgroup differences are not important enough in influence to change our conclusions regarding the effect size or are due to chance.
Additionally, we performed meta-regression subgroup analysis assessing whether dose of corticosteroid (as a continuous variable) had an effect on 28-day mortality. For this analysis, we used methylprednisone dose (mg/d) or equivalent, excluding the loading dose in studies that gave a larger initial day 1 corticosteroid dose. We hypothesized that there would be no significant difference between corticosteroid types, that early corticosteroid initiation would be more beneficial than late corticosteroid initiation, longer duration corticosteroid therapy would be more beneficial than shorter duration corticosteroid therapy, low-dose corticosteroid use would be superior to moderate–high dose corticosteroid use, that there would be no difference in effect between COVID-19 patients with ARDS and non COVID-19 patients with ARDS, and that studies with high ROB would show greater benefit with corticosteroids than low ROB studies. We also hypothesized that there would be no difference in studies conducted before and after 2015 or in studies that included formal ARDS criteria versus those that did not and that studies with a placebo would show a smaller effect than studies used usual care as comparator. Finally, we conducted sensitivity analyses excluding studies that reported mortality at endpoints other than 28 days and two additional posthoc sensitivity analyses requested by peer review excluding studies that did not explicitly have ARDS as part of their inclusion criteria and studies that initiated corticosteroids late.
We conducted TSA [17] using the random effects model for trials reporting mortality. For the TSA, we used a statistical significance level of 5%, a power of 80%, and a relative risk reduction (RRR) of 15% to represent a clinically important difference. Given the 20% risk of a false negative result with the first TSA, and at the request of peer reviewers, we also conducted a second posthoc sensitivity analysis TSA using a power of 90%. We performed TSA analysis using Trial Sequential Analysis version 0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, www.ctu.dk/tsa). For all other statistical analysis, we used RevMan 5.3 (Cochrane Collaboration, Oxford) software.
Results
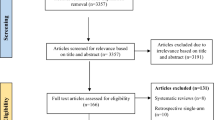
We included six RCTs (n = 833) from previously published meta-analyses [4, 10] addressing the topic [3, 18,19,20,21,22]. The updated search found 759 new citations, from which we included 12 additional RCTs (n = 1993) [7, 23,24,25,26,27,28,29] (e-Fig. 1) for a total of 18 RCTs (n = 2826) that met eligibility criteria. A recent meta-analysis published in JAMA [4] included seven RCTs examining the role of corticosteroids in patients with COVID-19. As not all of these patients were mechanically ventilated or had ARDS, we used data only from the subgroup of patients that received invasive mechanical ventilation (eFigure 1 from the JAMA report) or when not available, contacted individual trial authors for these subgroup results.
Table 1 shows characteristics of all included RCTs which randomized between 11 and 1007 patients. Five studies were conducted in the USA [18, 21, 23, 30], four in China [7, 22, 25, 26], two in Spain [3, 7], two in France [24], two in the UK [29], two in Brazil [27, 28], and one each in Denmark [7], Kuwait [20], Thailand [19], Australia [30], Canada [30], Ireland [30], the Netherlands [30], and New Zealand [30]. All patients included in the review were invasively ventilated, 12 of the RCTs included 1403 patients with AECC or Berlin-criteria ARDS [3, 7, 18,19,20,21,22,23,24,25,26,27], and 6 included 1423 patients with COVID-19 [7, 28, 29]. Additionally, while most non COVID-19 studies provided a breakdown of ARDS etiologies in their demographics, they did not provide outcome-based subgroup data based on specific aetiologies. Six trials used hydrocortisone [7, 19, 22, 24], eight used methylprednisolone [18, 20, 21, 23, 25, 26, 28], and four used dexamethasone [3, 7, 27, 29]. Two of the RCTs [21, 23] initiated corticosteroids late in the course of ARDS (defined as 1 week after diagnosis), whereas the other 16 RCTs initiated corticosteroids within the first week of diagnosis. Eight of the trials provided 7 days or less of corticosteroid therapy [7, 19, 22, 24,25,26, 28], while the rest provided 10 days or more. The median dose of corticosteroid used was 88 mg of methylprednisolone (equivalent to 400 mg of hydrocortisone) per day and seven of the included trials used a dose less than this [7, 19, 22, 24,25,26, 28, 29], while the other 11 used a dose higher than 88 mg. The lowest daily dose used was 30 mg of methylprednisolone [28, 29] while the highest was 120 mg of methylprednisolone [21, 23, 25, 26]. 10 RCTs administered a placebo to their control group [7, 18,19,20,21,22,23,24, 28] while the remainder only provided standard care.
We judged three RCTs to be at high ROB, one due to concerns regarding randomization and selection of the reported results [20] and another two due to incomplete reporting regarding randomization, descriptions of interventions, and selection of the reported results [25, 26]. The remainder of the trials were judged either at low ROB or some concern. e-Table 2 summarizes the ROB for each individual trial for the outcome of mortality. Table 2 and e-Table 3 depict the pooled outcomes with associated GRADE certainty of evidence.
Corticosteroids probably reduce 28-day mortality in patients with ARDS (2740 patients in 16 trials, RR 0.82, 95% CI 0.72–0.95, random effects model, absolute risk reduction (ARR) 8.0%, 95% CI 2.2–12.5% reduction, number needed to treat (NNT) 12.5, 95% CI 8.0–45.5, moderate certainty, Fig. 1). Both the initial TSA and posthoc TSA were consistent in that they showed that the optimal information size was not reached (n = 4690, 80% power; n = 6275, 90% power, Supplementary Materials).We rated this outcome down once due to a combination of borderline indirectness as eight of the studies included mechanically ventilated patients with COVID-19 respiratory failure, which were not explicitly defined as ARDS, and for borderline imprecision as although the 95% confidence interval only included benefit with corticosteroids, the optimal information size based on TSA was not met. When including trials that only enrolled patients meeting formal AECC/Berlin ARDS criteria, this conclusion and certainty of evidence did not change (1317 patients in 10 trials, RR 0.77, 95% CI 0.63–0.94, ARR 10.7%, 95% CI 2.8–17.3% reduction, NNT 9.3, 95% CI 5.8–35.7, moderate certainty, Fig. 2). Subgroup analysis based on COVID-19 status (Fig. 1), steroid type (e-Fig. 2), steroid initiation time (e-Fig. 3), steroid dosage (e-Fig. 4), and ROB (e-Fig. 5) did not demonstrate any credible subgroup effects. Meta-regression based on dosage of steroid as a continuous variable also showed no subgroup effect (p = 0.41, e-Fig. 6). Patients who received a longer course of corticosteroids (over 7 days) had higher rates of survival than those who received a shorter course (7 days or less) (p-value for subgroup interaction = 0.04, moderate credibility) (Fig. 3). We performed sensitivity analyses excluding the five studies that reported a mortality endpoint other than at 28 days [3, 20, 21, 23, 30] (e-Fig. 7), and the two studies that initiated corticosteroids late [21, 23] (e-Fig. 17), none of which substantially altered the pooled estimates or conclusions. None of our post-hoc subgroup analyses showed credible subgroup effects (e-Figs. 15, 16, 19).
Corticosteroid use may reduce ICU mortality (RR 0.61, 95% CI 0.38–0.99, ARR 18.6%, 95% CI 0.5–29.6% reduction, low certainty, e-Fig. 8) and probably reduce hospital mortality (RR 0.67, 95% CI 0.46–0.96, ARR 16.6%, 2.0–27.2% reduction, moderate certainty, e-Fig. 9) in critically ill patients with ARDS. The use of corticosteroids may lead to fewer days of mechanical ventilation (MD 4.04 days fewer, 95% CI 2.53–5.53 days fewer, low certainty, e-Fig. 18) and a shorter hospital length of stay (MD 8.05 days fewer, 95% CI 3.12–12.98 days fewer, low certainty, e-Fig. 10). There was an uncertain effect on ICU length of stay with corticosteroids (MD 0.78 days more, 95% CI 4.11 days more to 5.68 days fewer, very low certainty, e-Fig. 11).
There are unclear differences in rates of neuromuscular weakness (271 patients in 2 trials, RR 0.85, 95% CI 0.62–1.18, e-Fig. 12) and gastrointestinal bleeding (436 patients in 5 trials, RR 1.20, 95% CI 0.43–3.34, e-Fig. 13) with corticosteroids although these were all based on low or very low certainty evidence. There was probably an increase in hyperglycemia (915 patients in six trials, RR 1.11, 95% CI 1.01–1.23, moderate certainty evidence, e-Fig. 14) with corticosteroids; however, this outcome was rated down for indirectness, given the variability in definitions of hyperglycemia used across studies. Superinfections due to corticosteroid use are summarized as in e-Table 4. Since some studies individually counted multiple infections in one individual as separate data points and others did not, we opted not to pool this data. Although we could not pool data for this outcome, and understanding these limitations, it did not appear as though there was any signal for increase in superinfection (221 infections in corticosteroid group, 244 infections in control group).
Discussion
ARDS is in part the result of an innate immune-cell mediated inflammatory response that causes damage to the alveoli of the lung in response to a direct injury [9]. It has long been hypothesized that treatment with corticosteroids, as a potent anti-inflammatory agent, may benefit patients with ARDS, regardless of etiology [21, 23]. The results of our systematic review and meta-analysis is the first to support this hypothesis, indicating that corticosteroids may reduce mortality and the duration of mechanical ventilation in all patients with ARDS. Furthermore, corticosteroids likely cause few side effects, except for an increase in hyperglycemia. This effect on mortality appears to be consistent across COVID-19 ARDS (regardless of strict ARDS criterion) and non-COVID-19 patients, and between corticosteroid type, timing and dose, although a longer duration of therapy may be more beneficial compared to a shorter course. Given the consistency of the results between ARDS etiology, this analysis supports the hypothesis that corticosteroids should be considered in all patients with ARDS, assuming no contraindications.
The 2017 SCCM and ESICM guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients [31] made a conditional recommendation for corticosteroid use in patients with ARDS and a PaO2/FiO2 ratio < 200. Prior meta-analyses examining this topic [4, 10, 32] have also demonstrated a consistent finding of decreased mortality and decreased duration of mechanical ventilation with corticosteroid when used in patients with ARDS. Now with the addition of COVID-19 RCTs, estimates of effect in ARDS are more precise and based on a larger number of patients. In addition, more comprehensive subgroup analyses are possible in order to address lingering questions regarding populations of interest and optimal steroid administration regimes. Unfortunately, while no definitive conclusions could be made, this meta-analysis did indicate that longer duration corticosteroids may be more beneficial than a shorter course. Furthermore, while we cannot comment on late administration of corticosteroids given that almost all our included RCTs initiated corticosteroids within the first week of ARDS diagnosis, starting corticosteroids more than 2 weeks after ARDS diagnosis may be harmful, as illustrated by Steinberg et al. [21] as the exudative/inflammatory phase of ARDS has passed. Given the findings of this updated review, clinical practice guidelines addressing ARDS, including the SCCM/ESICM CIRCI guideline, will need to be re-evaluated. Despite the increased clarity, residual questions remain regarding the most beneficial corticosteroid regime. In addition, limitations of trial level data do not allow for a more granular assessment beyond considering single subgroups at once when it is possible that multiple variables could explain the differences or lack of differences in effect sizes. Conducting an individual patient level meta-analysis may allow for a more comprehensive exploration of these subgroups of interest. Specific factors affecting the response to corticosteroid treatment in patients with ARDS were the subject of a recent review addressing the role of dosage, timing of initiation, mode of administration, duration, and tapering in achieving optimal response to corticosteroid treatment in ARDS [33].
Recent RCTs examining the treatment of COVID-19 respiratory failure have shown consistent benefit with corticosteroids, especially in the critically ill [7]. This has led to multiple clinical practice guidelines strongly recommending the use of corticosteroids for the treatment of critically ill COVID-19 patients, including those on mechanical ventilation [8, 34]. Although still relatively early in our understanding of COVID-19 ARDS, most treatment regimes for COVID-induced ARDS mimic those of non-COVID-ARDS including low tidal volumes, optimal positive end expiratory pressure (PEEP), and prone positioning for severe cases [35]. The results of this review further demonstrate the consistency of ARDS when it comes to treatment. While six of the included trials did not include patients who met Berlin/AECC criteria for ARDS, the similarity of treatment effect and lack of subgroup differences between studies that meet strict criteria and studies that do not suggest that patients in all these studies can be treated similarly in the context of corticosteroids. Furthermore, given the somewhat arbitrary and imprecise nature of the AECC/Berlin criteria [36], our study suggests that following the strict definition criteria itself to inform treatment with corticosteroids of patients with ARDS may miss patients who may benefit from this therapy. Additionally, we have demonstrated that corticosteroids have very similar effects on mortality in both the COVID-19 ARDS subgroup (RR 0.89, 95% CI 0.76–1.05) and the non-COVID-19 ARDS (RR 0.70, 95% 0.55–0.89). This consistency suggests that COVID-19 ARDS may be treated similarly to non-COVID-19 ARDS when it comes to corticosteroids.
This is the largest and most comprehensive meta-analysis to date examining corticosteroids in ARDS of any cause. Furthermore, unlike previous meta-analyses [4, 10, 11], this is the only review to combine both COVID − ARDS and COVID + ARDS patients in the same meta-analysis. Strengths of this review include the comprehensive search, pre-registration of the protocol, careful evaluation of subgroups of interest including COVID-19 versus non-COVID, and including meta-regression to evaluate the impact of corticosteroid dose, assessment of ROB using Cochrane 2.0, TSA and certainty of evidence using the GRADE approach. This review also has limitations. First, as mentioned above, while most studies included patients with ARDS as defined by the AECC [12] or Berlin criteria [13], 6 RCTs of COVID did not strictly enroll patients with ARDS as per Berlin. Given that patients with COVID-19 respiratory failure significant enough to require invasive mechanical ventilation usually present with bilateral infiltrates [37], we felt that this population was similar enough to the ARDS population to be included. It is possible that we may have missed patients that would have met ARDS criteria but who were not intubated, e.g. those on non-invasive ventilation or included patients who did not meet ARDS criteria e.g. COVID-19 patients without bilateral infiltrates or PF < 200; however, this would likely represent a small proportion of total ARDS patients. Further, when we conducted analyses excluding all trials that did not meet strict AECC/Berlin criteria or separated these trials out as their own subgroup, treatment effect sizes remained similar, there was a lack of significant subgroup effects and the overall certainty of our conclusions remained unchanged. However, we recognize that the lack of formal ARDS criteria for all studies provides a potential source of indirectness in our results and have accordingly downgraded the certainty of outcomes to reflect this. We also recognize that not all studies used the same criteria to define ARDS, with some studies using the AECC criteria and others using the Berlin criteria. However, as we only included patients that required mechanical ventilation, studies that used the Berlin criteria only included patient with moderate-to-severe ARDS [3, 7, 27]. This makes the criteria (AECC and Berlin) functionally the same for the purposes of this review. Second, the underlying etiology of ARDS in these studies was heterogenous and most non-COVID studies were small. However, it is reassuring that the effect of corticosteroids was consistent across included studies (regardless of etiology or size of the trial) with low levels of statistical heterogeneity. Some of the trials included in this meta-analysis are older, including one which is more than 20 years old [23], and since then, standard of care has changed; however, again we did not observe high degrees of statistical heterogeneity to suggest this may have influenced the certainty of our results. Moreover, when using the GRADE methodology to rate outcomes, we rated importance of outcomes through consensus of the co-author group. In an ideal world, a wider survey including actual patients would have provided a more robust rating. Finally, we were unable to find evidence addressing long-term survival, as well as data on children or in low and middle income countries—all important areas for future research.
Conclusion
The use of corticosteroids probably reduces mortality and may reduce the duration of mechanical ventilation in patients with ARDS. This effect was consistent between patients with COVID-19 and non-COVID-19 ARDS and between different corticosteroid types, and dosage.
Data availability
Available upon request.
References
Prescott HC, Rice TW (2020) Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. https://doi.org/10.1001/jama.2020.16747
Annane D, Renault A, Brun-Buisson C et al (2018) Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 378:809–818. https://doi.org/10.1056/NEJMoa1705716
Villar J, Ferrando C, Martínez D et al (2020) Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 8:267–276. https://doi.org/10.1016/S2213-2600(19)30417-5
Ye Z, Wang Y, Colunga-Lozano LE et al (2020) Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ 192:E755–E767. https://doi.org/10.1503/cmaj.200645
Weile J, Streeck B, Muck J et al (2009) Severe cytomegalovirus-associated esophagitis in an immunocompetent patient after short-term steroid therapy. J Clin Microbiol 47:3031–3033. https://doi.org/10.1128/JCM.00143-09
Kumar A, Paulose R, Sadasivan S et al (2020) Sarcoidosis, steroids and Strongyloides—what’s the catch? Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2020.09.012
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S et al (2020) Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. https://doi.org/10.1001/jama.2020.17023
Lamontagne F, Agoritsas T, MacDonald H et al (2020) A living WHO guideline on drugs for covid-19. BMJ. https://doi.org/10.1136/bmj.m3379
Thompson BT, Chambers RC, Liu KD (2017) Acute respiratory distress syndrome. N Engl J Med 377:562–572. https://doi.org/10.1056/NEJMra1608077
Mammen MJ, Aryal K, Alhazzani W, Alexander PE (2020) Corticosteroids for patients with acute respiratory distress syndrome: a systematic review and meta-analysis of randomized trials. Polish Arch Intern Med 130:276–286
Lamontagne F, Briel M, Guyatt GH et al (2010) Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care 25:420–435. https://doi.org/10.1016/j.jcrc.2009.08.009
Bernard GR, Artigas A, Brigham KL, et al (1994) The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. In: American Journal of Respiratory and Critical Care Medicine. American Thoracic Society, pp 818–824
Ranieri VM, Rubenfeld GD, Thompson BT et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533. https://doi.org/10.1001/jama.2012.5669
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Chapter 6: Choosing effect measures and computing estimates of effect | Cochrane Training. https://training.cochrane.org/handbook/current/chapter-06#_Ref186713714. Accessed 14 Sep 2020
Schandelmaier S, Briel M, Varadhan R et al (2020) Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 192:E901–E906. https://doi.org/10.1503/cmaj.200077
Wetterslev J, Jakobsen JC, Gluud C (2017) Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 17:39. https://doi.org/10.1186/s12874-017-0315-7
Meduri GU, Golden E, Freire AX et al (2007) Methylprednisolone infusion in early severe ards: results of a randomized controlled trial. Chest 131:954–963. https://doi.org/10.1378/chest.06-2100
Tongyoo S, Permpikul C, Mongkolpun W et al (2016) Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care 20:1–11. https://doi.org/10.1186/s13054-016-1511-2
Abdelsalam Rezk N, Mohamed Ibrahim A (2013) Effects of methyl prednisolone in early ARDS. Egypt J Chest Dis Tuberc 62:167–172. https://doi.org/10.1016/j.ejcdt.2013.02.013
Steinberg KP, Hudson LD, Goodman RB et al (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684. https://doi.org/10.1056/NEJMoa051693
Liu L, Li J, Huang Y-z et al (2012) The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency. Zhonghua Nei Ke Za Zhi 51:599–603
Meduri GU, Headley AS, Golden E et al (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. J Am Med Assoc 280:159–165. https://doi.org/10.1001/jama.280.2.159
Annane D, Sébille V, Bellissant E (2006) Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 34:22–30. https://doi.org/10.1097/01.CCM.0000194723.78632.62
Zhi-fang S, Li-jun Y (2016) Effect of glucocorticoid on extravascular lung water in the patients with acute respiratory distress syndrome. Chinese J Crit Care Med 36:443–447
Zhou M (2015) Application value of glucocorticoids in comprehensive treatment of acute respiratory distress syndrome caused by severe community-acquired pneumonia. Clin Med Eng 22:57–58
Tomazini BM, Maia IS, Cavalcanti AB et al (2020) Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2020.17021
Jeronimo CMP, Farias MEL, Val FFA et al (2020) Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1177
RECOVERY Collaborative Group, Horby P, Shen Lim W et al (2020) Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. https://doi.org/10.1056/nejmoa2021436
Angus DC, Berry S, Lewis RJ et al (2020) The remap-cap (Randomized embedded multifactorial adaptive platform for community-acquired pneumonia) Study rationale and design. Ann Am Thorac Soc 17:879–891. https://doi.org/10.1513/AnnalsATS.202003-192SD
Annane D, Pastores SM, Rochwerg B et al (2017) Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I). Crit Care Med 45:2078–2088. https://doi.org/10.1097/CCM.0000000000002737
Meduri GU, Siemieniuk RAC, Ness RA, Seyler SJ (2018) Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care 6:53
Meduri GU, Annane PD, Confalonieri M et al (2020) Pharmacological principles guiding prolonged glucocorticoid treatment In ARDS. Intensive Care Med 46(12):2284–2296
Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 11 Oct 2020
Fan E, Beitler JR, Brochard L et al (2020) COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 8:816–821
Tobin MJ (2020) Does making a diagnosis of ARDS in patients with coronavirus disease 2019 matter? Chest 158:2275–2277
Bos LDJ, Brodie D, Calfee CS (2020) Severe COVID-19 infections—knowledge gained and remaining questions. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.6047
Author information
Authors and Affiliations
Contributions
DC, BR and GUM came up with the idea for the study. DC, MJM, ZY, PA, LL and SS performed study screening. DC, KS, ZY, LL, MJM and AK performed dual data abstraction. BR adjudicated for any potential disagreements. DC performed the statistical analysis. LM provided statistical assistance with the meta-regression. KL, MWM, AP, BD, WA, SP, JM, FL, DA, DC and BR helped write the first draft. All other authors helped with editing the subsequent manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Conflicts of interest
AP is the sponsor and MWM the coordinating investigator of the COVID STEROID trial, which is funded by the Novo Nordisk Foundation and supported by Pfizer Inc. WA is the chair of the Surviving Sepsis COVID guideline. BR was the methodologist for the 2017 CIRCI SCCM guidelines and on the methods team for the WHO corticosteroid in COVID guideline. SP served as co-chair of the SCCM/ESICM 2017 Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients. DA has been the co-chair of the SCCM/ESICM guidelines on the diagnosis and management of CIRCI 2017 (PMID: 29095205; PMID: 29090327; PMID: 28940017; PMID: 28940011; PMID: 28938253; PMID: 28938251). He was a member of WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group which has published a prospective meta-analysis on the use of corticosteroids for COVID-19 (PMID: 32876694; PMID: 32831155); and a member of the steering committee of BMJ/GRADE rapid recommendations for corticosteroids for sepsis (PMID: 30097460; PMID: 29979221). DA is coordinating the individual patient data meta-analysis on hydrocortisone for septic shock (PMID: 33268422) and has coordinated the Cochrane trial-level meta-analysis on the use of corticosteroids for children and adults with sepsis (PMID: 31808551). DA has been the PI of the following trials on corticosteroids for sepsis (and or ARDS) PMID: 20103758; PMID: 18184957; PMID: 16374152; PMID: 12186604; PMID: 29490185. DA was the member of the steering committee of the following trials of corticosteroids for COVID-19: PMID: 32876697; PMID: 32876689. DA has received no personal rewards for any of the above mentioned academic activities. LM does paid consulting work for Bayer (oncology), AstraZeneca (biologics for asthma) and Janseen (TB drugs). GUM served as a member of the SCCM/ESICM 2017 Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients. MJM serves as a co-author on the Surviving Sepsis COVID guideline. FL contributed to systematic reviews and clinical trials of evaluating the effects of corticosteroids in ARDS and pneumonia and chaired the WHO corticosteroid in COVID guideline.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaudhuri, D., Sasaki, K., Karkar, A. et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med 47, 521–537 (2021). https://doi.org/10.1007/s00134-021-06394-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06394-2