- 1Department of Neurology, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Neurology and Stroke Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 3Department of Rehabilitation, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 4Department of Ideological and Political Theory Teaching, Maoming Polytechnic, Maoming, China
- 5Department of Rehabilitation, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China
Background: COVID-19 is a type of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that was identified in December 2019. Corticosteroid therapy was empirically used for clinical treatment in the early stage of the disease outbreak; however, data regarding its efficacy and safety are controversial. The aim of this study was to evaluate the efficacy and safety of corticosteroid therapy in patients with COVID-19.
Methods: The PubMed, Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang, and China Science and Technology Journal (VIP) databases were searched for studies. Data on clinical improvement, mortality, virus clearance time, adverse events (AEs), utilization of mechanical ventilation, length of intensive care unit (ICU) hospitalization, and hospital stay were extracted by two authors independently. Study quality was assessed by the Newcastle Ottawa Scale (cohort studies). The pooled data were meta-analyzed using a random effects model, and the quality of evidence was rated using the GRADE approach.
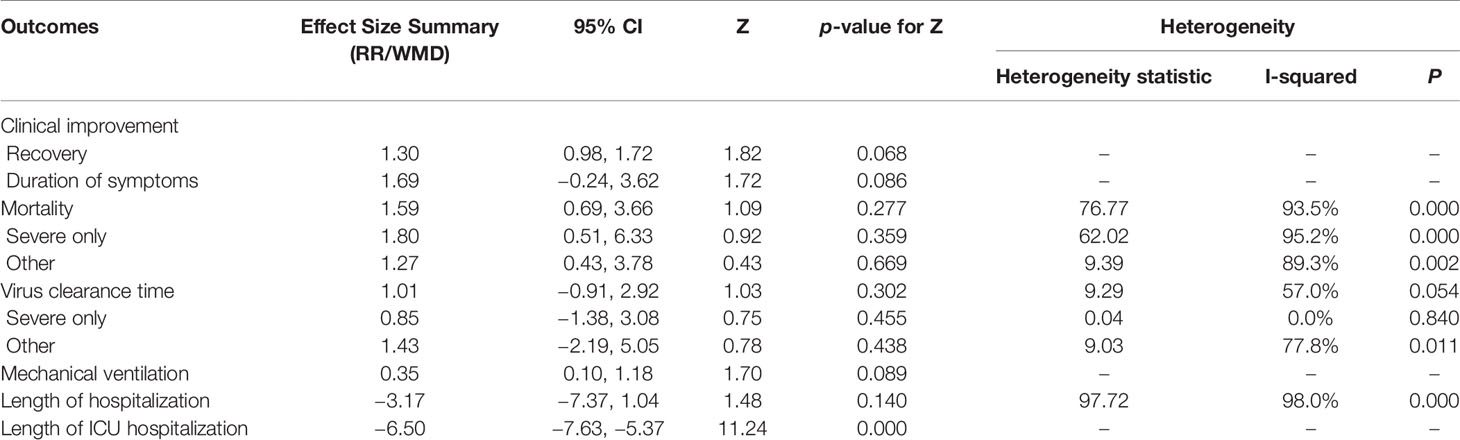
Results: Eleven cohort studies (corticosteroid group vs control group), two retrospective cohort studies (without control group), and seven case studies were identified. A total of 2840 patients were included. Compared with the control treatments, corticosteroid therapy was associated with clinical recovery (RR = 1.30, 95% CI [0.98, 1.72]) and a significantly shortened length of ICU hospitalization (RR = −6.50; 95% CI [−7.63 to −5.37]), but it did not affect the mortality ((RR = 1.59; 95% CI [0.69–3.66], I2 = 93.5%), utilization of mechanical ventilation (RR = 0.35; 95% CI [0.10, 1.18]), duration of symptoms (WMD = 1.69; 95% CI [−0.24 to 3.62]) or virus clearance time (RR = 1.01; 95% CI [−0.91 to 2.92], I2 = 57%) in COVID-19 patients. Treatment with corticosteroids in patients with COVID-19 may cause mild adverse outcomes. The quality of evidence was low or very low for all outcomes.
Conclusion: The findings of our study indicate that corticosteroid therapy is not highly effective, but it appears to improve prognosis and promote clinical recovery in patients with severe COVID-19.
Introduction
Since COVID-19 was first discovered in Wuhan, Hubei Province, China, in December 2019, confirmed and suspected cases have been reported in other parts of China and abroad (Wang C. et al., 2020). The outbreak has now reached a pandemic level, with a high global mortality rate (Baud et al., 2020). It has attracted global attention because of its potential for widespread human infection and economic loss. Although the characteristics and pathogenesis of COVID-19 are gradually being revealed, the efficacy and safety of corticosteroid use in patients remain controversial (Russell et al., 2020; Zhong et al., 2020).
Immunological studies have shown that the higher concentration of cytokines and chemokines were detected in patients with COVID-19, and the release of excessive proinflammatory cytokines can promote patients to ARDS, multiple organ dysfunction, and death (Isidori et al., 2020; Shi et al., 2020; Yang J. et al., 2020). Immune suppression is a typical function of corticosteroids (Sehgal and Malhotra, 2019), and treatment with corticosteroids in COVID-19 patients might improve severe clinical symptoms and reduce mortality. Moreover, for coronavirus pneumonia (such as SARS and MERS, caused by SARS-CoV and MERS-CoV, which is similar SARS-CoV-2), corticosteroids are the main anti-inflammatory therapy (Zhong and Zeng, 2003; Arabi et al., 2017), and numerous clinical studies have reported the efficacy of corticosteroids in the treatment of these diseases (Zhao et al., 2003; Chen et al., 2006). Based on the above evidence, it is possible that corticosteroid therapy may be effective in patients with COVID-19.
Some clinical studies also showed that corticosteroid therapy was effective in patients with COVID-19. A retrospective review was conducted by Wang et al. to explore the efficacy of the early use of short-term corticosteroids compared with a control treatment in hospitalized patients with severe COVID-19 pneumonia in Wuhan Union Hospital and reported a remarkable improvement of clinical symptoms and chest computed tomography (CT) findings (Wang et al., 2020a). Moreover, in a cohort study involving 201 patients with confirmed COVID-19 pneumonia, the authors found that the use of methylprednisolone notably reduced the risk of death (Wu C. et al., 2020). Furthermore, China’s “Diagnosis and Treatment Protocol for COVID-19 (Trial Seventh Edition)” also mentions that different doses and treatment courses of methylprednisolone are recommended for general (with high-risk factors for severe COVID-19), severely ill, and critically ill patients with COVID-19 pneumonia to prevent inflammation and reduce exudation (General Office of National Health Commission of People’s Republic of China, 2020). All of these findings seem to indicate the effectiveness of corticosteroids in the treatment of COVID-19.
However, the interim guidelines from the WHO on the clinical management of severe acute respiratory infection when novel coronavirus (SARS-CoV-2) infection is suspected do not advise the use systemic corticosteroids (Organization, 2020); other clinical trials have also indicated that the use of corticosteroids in patients with COVID-19 may not be a good choice. A retrospective, single-center case series including 138 consecutive hospitalized patients conducted by Wang et al. reported no effective outcomes in 44.9% of COVID-19 patients treated with glucocorticoids (Wang D. et al., 2020). Similar results were observed in Kui’s study. The recently published retrospective study by Kui et al. reported no beneficial effect of the use of methylprednisolone on clinical outcomes (Kui et al., 2020). Moreover, previous studies have shown inconclusive clinical evidence of the effect of corticosteroid therapy for viral pneumonia (such as SARS, MERS, and H1N1), and pulse-dose therapy or long-term use of a high-dose corticosteroid in the early stage of disease might be harmful (Stockman et al., 2006; Arabi et al., 2017; Moreno et al., 2018).
Given this background, the effectiveness of corticosteroid therapy for COVID-19 urgently needs to be evaluated. Two recently published articles have reviewed and summarized this issue and reported inadequate evidence to support the routine use of corticosteroids in COVID-19 patients (Li et al., 2020; Veronese et al., 2020). However, one of the two reviews included only four retrospective studies because of its early publication date (Veronese et al., 2020), and the result of the other review was based on disease caused by similar viruses (SARS-CoV and MERS-CoV) but not COVID-19 specifically (Li et al., 2020). Therefore, the results of these published reviews might be inaccurate, and the effects of corticosteroid therapy in COVID-19 patients remain unclear.
In this study, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of the use of corticosteroids in patients with COVID-19. All the types of literature currently available were included, including randomized controlled trials (RCTs), prospective or retrospective studies, case series, and case reports. The eligible RCTs and cohort studies were used for quantitative synthesis (meta-analysis), and the remaining types of studies were used for qualitative synthesis.
Methods
The present study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and a previously published protocol (PROSPERO: CRD42020184545) (Moher et al., 2015).
Search Strategy
The electronic databases PubMed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), WANFANG DATA, and China Science and Technology Journal Database (VIP) were searched for studies. The search terms were “COVID-19 OR 2019 novel coronavirus disease OR SARS-CoV-2 OR 2019-nCoV OR COVID-19 pandemic AND corticosteroid OR glucocorticoid OR steroid OR methylprednisolone OR hydrocortisone OR prednisone.” We manually searched for further articles by tracing the references included in the articles. The database search was run from database inception until 30 July 2020. One reviewer first evaluated the literature to select the studies based on the title and abstract, followed by reading the full text of the remaining reports.
Study Selection and Outcomes
Studies were selected according to the following inclusion criteria: (1) patients diagnosed with COVID‐19, regardless of severity; (2) patients treated with corticosteroids; (3) patients assessed the efficacy or safety of corticosteroids; (4) because of the scarcity of literature, no restriction on study type or study design (be either a randomized controlled trial, prospective or retrospective study, case series or case report); and (5) published in English and/or Chinese.
The primary outcomes of this study included clinical improvement (recovery and duration of symptoms), mortality, virus clearance time, and adverse events (AEs). The secondary outcomes included utilization of mechanical ventilation, length of intensive care unit (ICU) hospitalization and hospital stay, and discharge data.
Data Collection and Extraction
Data were independently extracted by two investigators using a standardized data-recording form, and disagreements between authors were discussed with corresponding author. The following information was extracted from the included studies: (1) study characteristics: 1st author, year of publication, study design, study region/country, and sample size; (2) population characteristics: mean/median age, sex, degree of severity, and original comorbidities; (3) corticosteroid treatment: the corticosteroid type, dosage, and duration; and (4) outcomes: primary and secondary outcomes. We applied a Java program called Plot Digitizer (http://plotdigitizer.sourceforge.net/) to convert plotted values into numerical form if adequate information was not provided by the study. The corresponding authors were contacted if the data of interest were not reported.
Quality Assessment
All eligible studies were assessed by two other authors independently. A third reviewer arbitrated in cases of disagreement. The methodological quality of prospective or retrospective studies was assessed by the New-castle Ottawa Scale (NOS) considering three domains: subject selection, comparability of the study groups, and assessment of outcome (Wells et al.). A NOS score ≥7 was considered high quality. The quality of evidence was assessed with the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach and was graded as high, moderate, low, or very low (Meader et al., 2014).
Data Synthesis and Statistical Analysis
Data analyses were conducted using STATA 12.0 software. The effect sizes were estimated by risk ratios (RRs) with 95% confidence intervals for dichotomous data and weighted mean differences (WMDs) with 95% confidence intervals for continuous data. Due to differences in sample characteristics and the small number of studies included, a random effects model was chosen to pool the effect sizes. The I2 statistic was applied to measure heterogeneity among the studies in each analysis, and we considered an I2>50% as notable heterogeneity. Subgroup analysis was performed according to the severity of disease, including severe only and other. Only cohort studies (corticosteroid group vs control group) were used for quantitative synthesis, and eligible retrospective cohort studies (without control group) and case studies were used for qualitative synthesis. In addition, if the reported results of the included studies were median and range, and the author that be contacted to request the original data cannot be recovered, we would convert the median and range into mean and standard deviation through the data conversion method applied by Hozo et al (Hozo et al., 2005).
Results
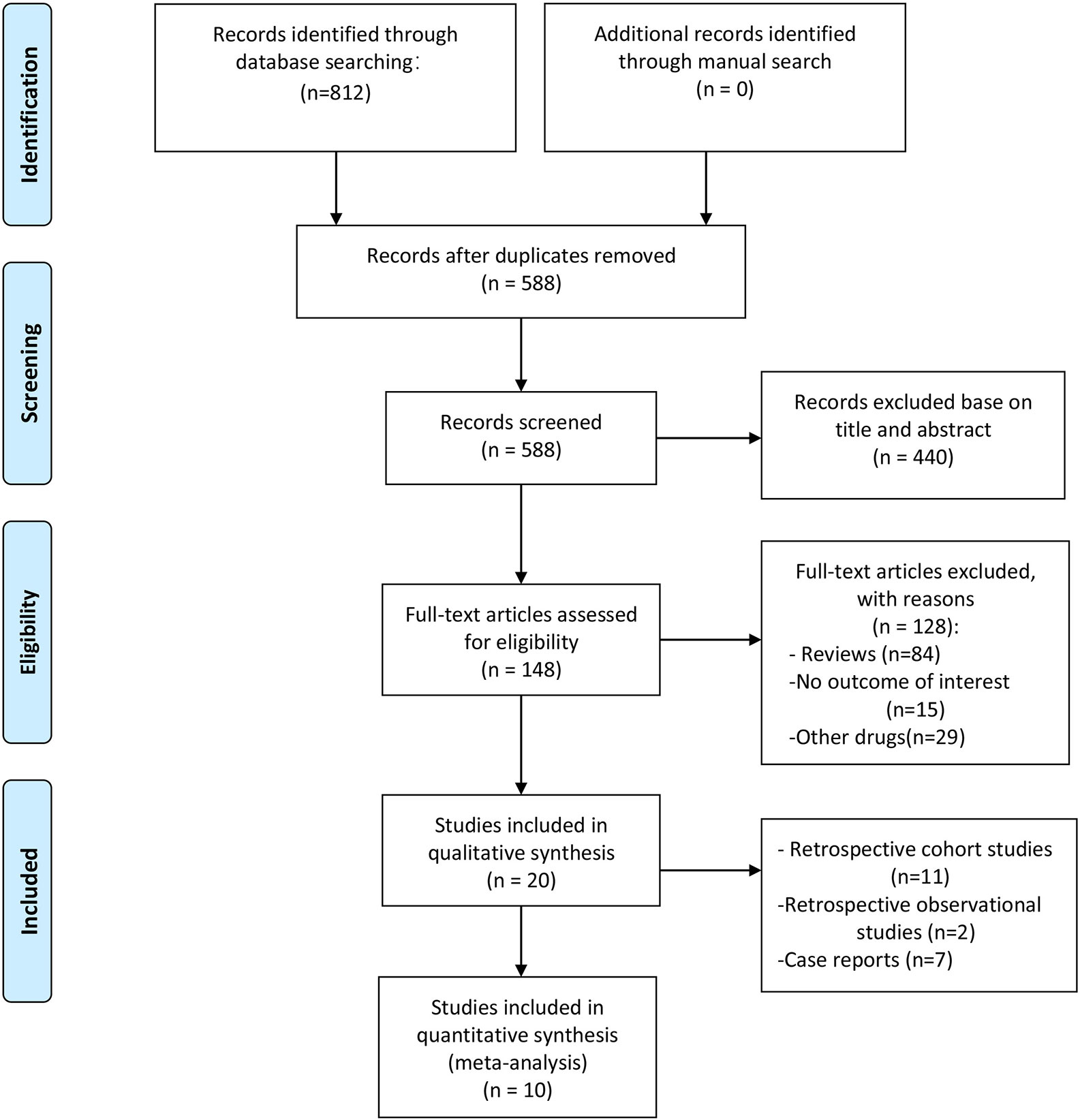
The initial literature search identified 812 publications. After duplicate publication removal and screening of titles and abstracts, 664 citations were excluded, and 148 studies were selected for a full-text review. Following application of the eligibility criteria, 20 articles with 2840 patients met the inclusion criteria. A summary of the literature search according to the PRISMA flowchart is presented in Figure 1.
Characteristics of the Included Studies
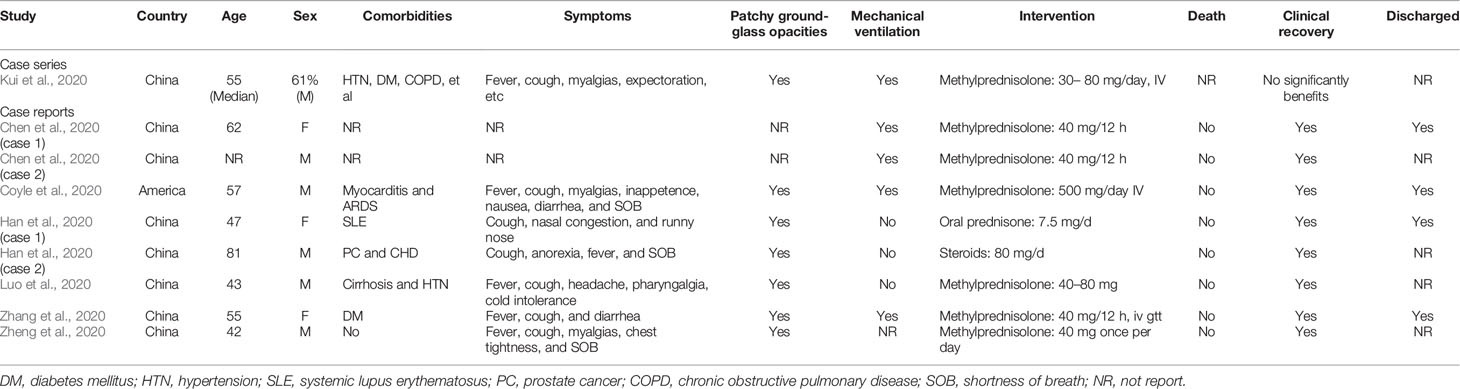
This study included 20 studies for qualitative synthesis. Eleven of these 20 studies were cohort studies (corticosteroid group vs control group) (Fadel et al., 2020; Fang et al., 2020a; Fang et al., 2020b; Ling et al., 2020; Lu et al., 2020; Ni et al., 2020; Wang et al., 2020b; Wu C. et al., 2020; Wu J. et al., 2020; Yang X. et al., 2020; Zha et al., 2020; Zhou F. et al., 2020), and two were retrospective cohort studies (without control group) (Li and Liu, 2020; Zhou W. et al., 2020), while the remaining 7 publications were case studies (Chen et al., 2020; Coyle et al., 2020; Han et al., 2020; Kui et al., 2020; Luo et al., 2020; Zhang et al., 2020; Zheng et al., 2020). In five studies, the publication language was Chinese (Li and Liu, 2020; Luo et al., 2020; Ni et al., 2020; Zhang et al., 2020; Zheng et al., 2020), and in the others it was English. Two study was conducted in America (Coyle et al., 2020; Fadel et al., 2020), and the eighteen other studies were conducted in China. The intervention in the eligible studies was corticosteroid therapy, including methylprednisolone (12 studies), corticosteroid (5 study), glucocorticoids (1 study), methylprednisolone sodium succinate (1 study), steroids (1 study), and prednisone (1 study).
Summaries of the demographic and clinical characteristics of the included cohort studies (corticosteroid group vs control group), retrospective cohort studies (without control group) and case studies are shown in Tables 1–3, respectively. Summary of the interventions and outcomes of the included cohort studies (corticosteroid group vs control group) and retrospective cohort studies (without control group) are showed in Tables 4 and 5, respectively.
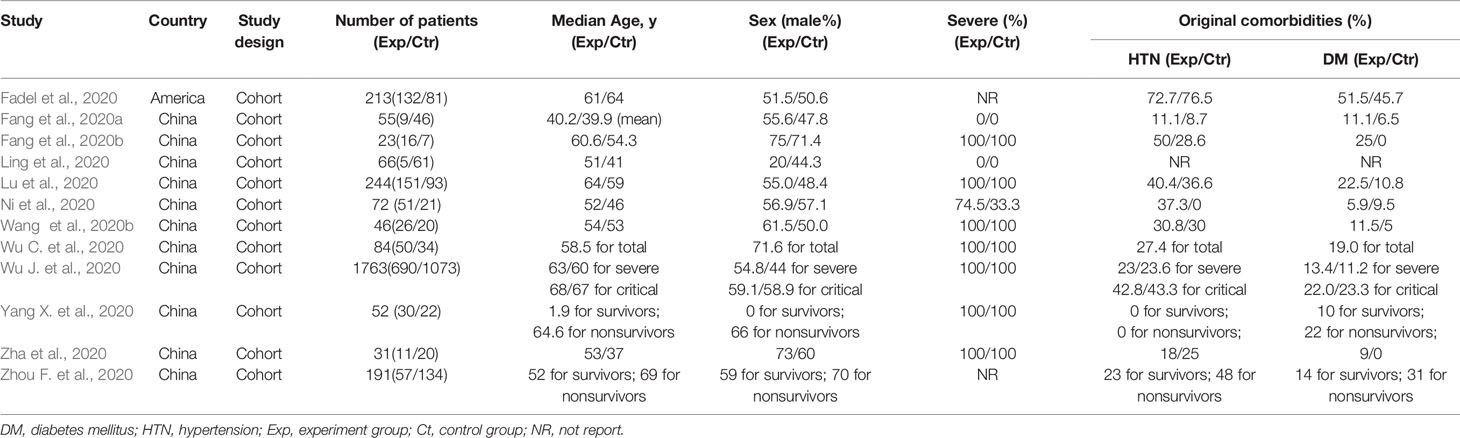
Table 1 Summary of the demographic and clinical characteristics of the included cohort studies (corticosteroid group vs control group).

Table 2 Retrospective observational studies involving the use of corticosteroids in patients with COVID-19 (without control group).
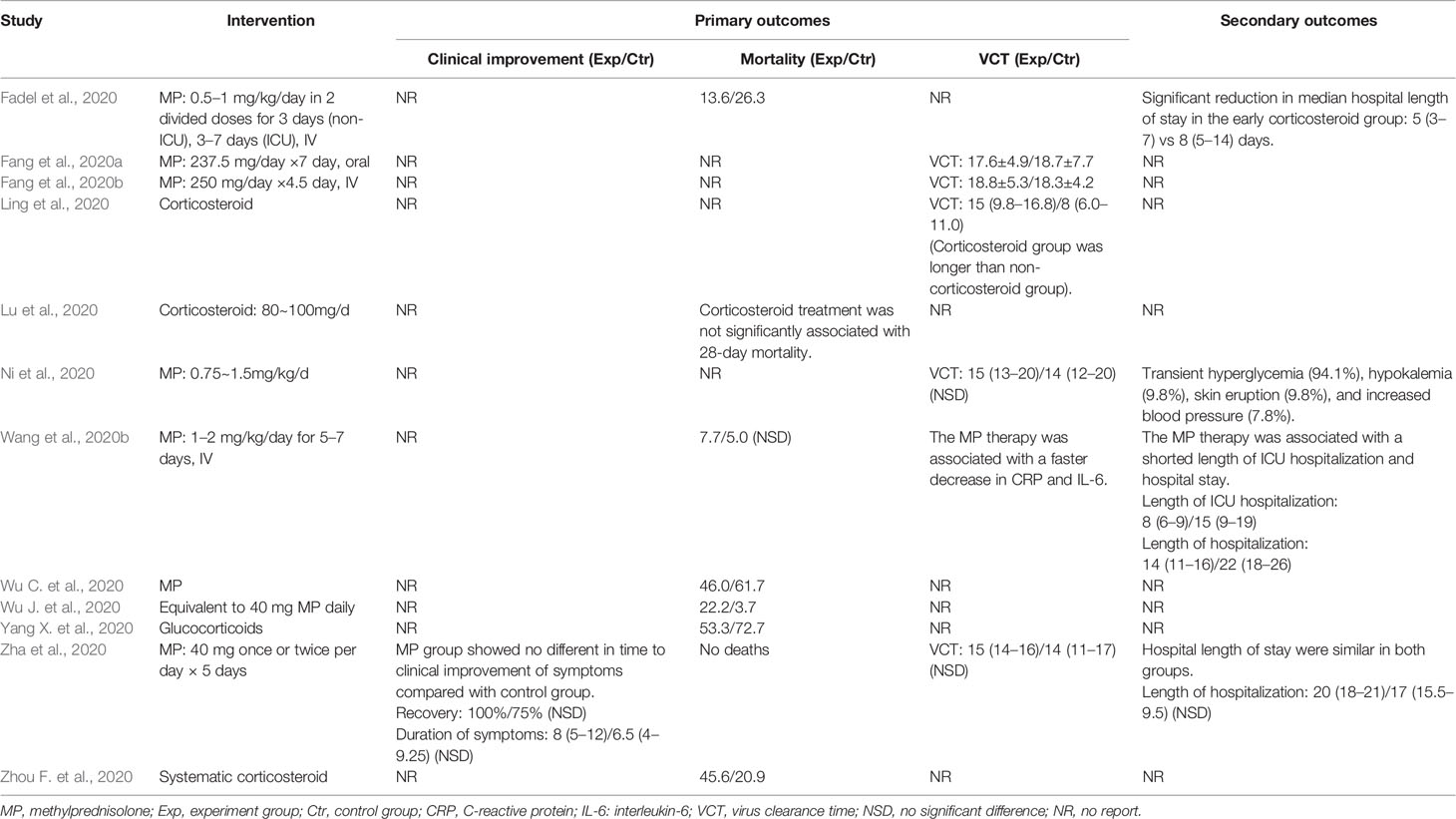
Table 4 Summary of the interventions and outcomes of the included cohort studies (corticosteroid group vs control group).
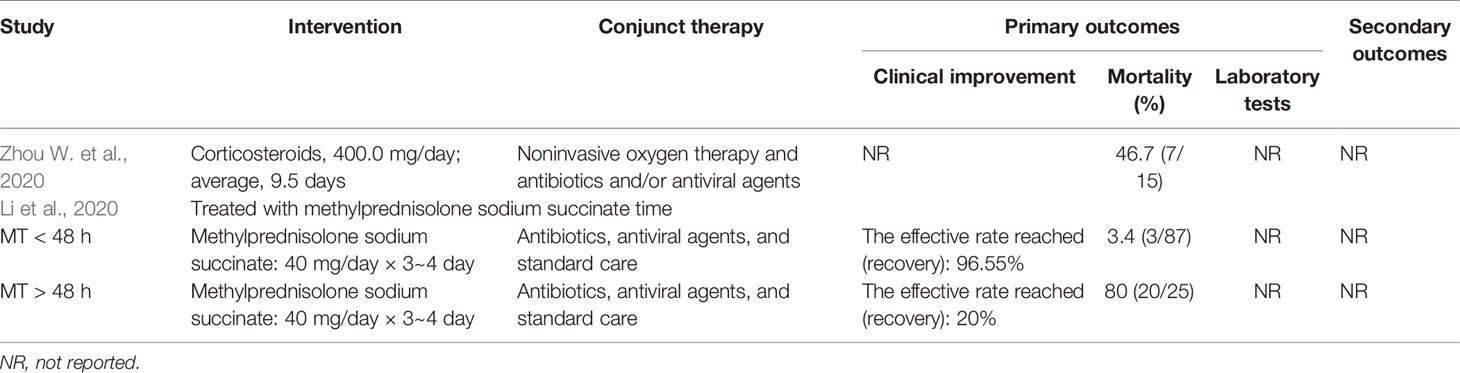
Table 5 Summary of the interventions and outcomes of the included retrospective studies (without control group).
Methodological Quality of the Included Studies
The meta-analysis included only cohort studies (corticosteroid group vs control group). One of the cohort studies (corticosteroid group vs control group) was excluded because it did not report numerical data of interest (Lu et al., 2020). Finally, ten cohort studies (corticosteroid group vs control group) were included in the quantitative synthesis. The NOS scores of the ten cohort studies ranged from 6 to 9, indicating that most of the eligible cohort studies had a low risk of bias (Table 6).
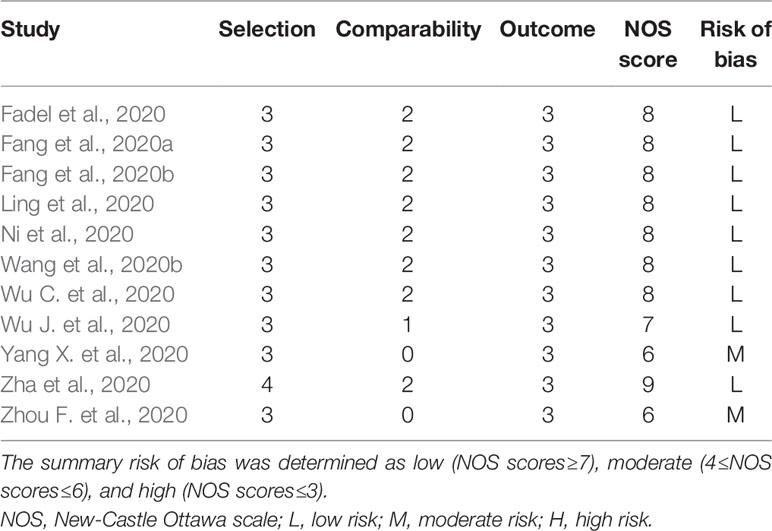
Table 6 Risk of bias summary: the review authors’ judgments about each risk-of-bias item for each included cohort study (corticosteroid group vs control group).
Outcomes
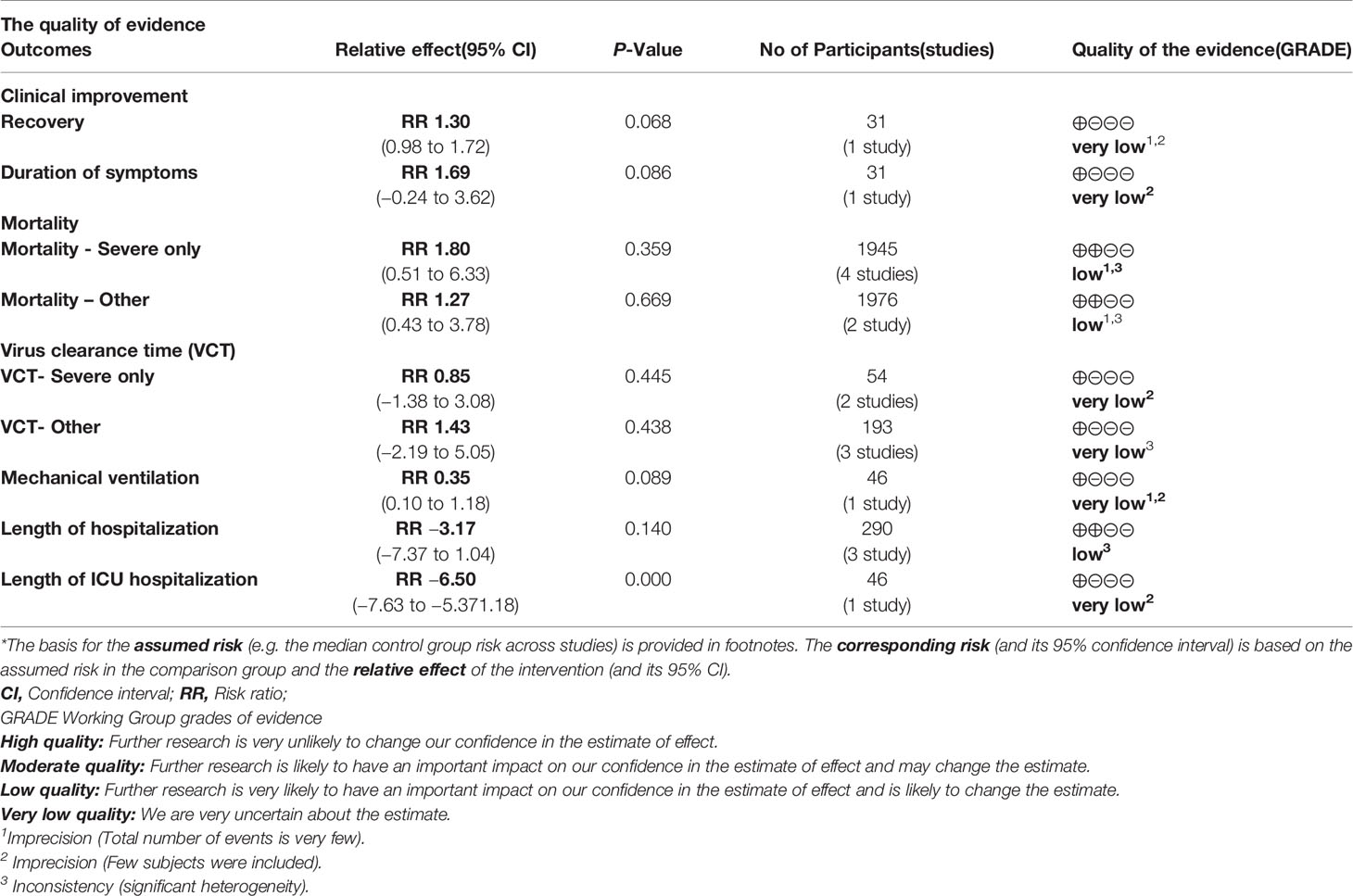
Summary of the meta-analysis results are shown in Table 7.
Clinical Improvement
Among the cohort studies (corticosteroid group vs control group), only one study that compared 31 patients (11 in the corticosteroid treatment group and 20 in the control group) was included in the meta-analysis (Zha et al., 2020). Corticosteroid treatment did not significantly shorten the duration of symptoms (WMD = 1.69, 95% CI [−0.24 to 3.62]) (Table 7). The meta-analysis revealed a tendency of significantly higher recovery rate in the corticosteroid group than in the control group (RR = 1.30, 95% CI [0.98, 1.72]) (Table 7).
One out of two included retrospective cohort studies (without control group) reported the outcome of clinical improvement (Li and Liu, 2020). Li and colleagues retrospectively studied the clinical data of 112 cases treated with low-dose methylprednisolone sodium succinate when mild and noncritical cases became severe and critical and reported that the recovery rates from aggravation of COVID-19 within 48 h and after 48 h were 96.55% and 20%, respectively (Table 5). Among the included case studies, only one study reported that there was no beneficial effect of the use of methylprednisolone on clinical improvement (Kui et al., 2020). The remaining studies showed that all patients had recovered from COVID-19 at the time of publication (Table 3).
Mortality
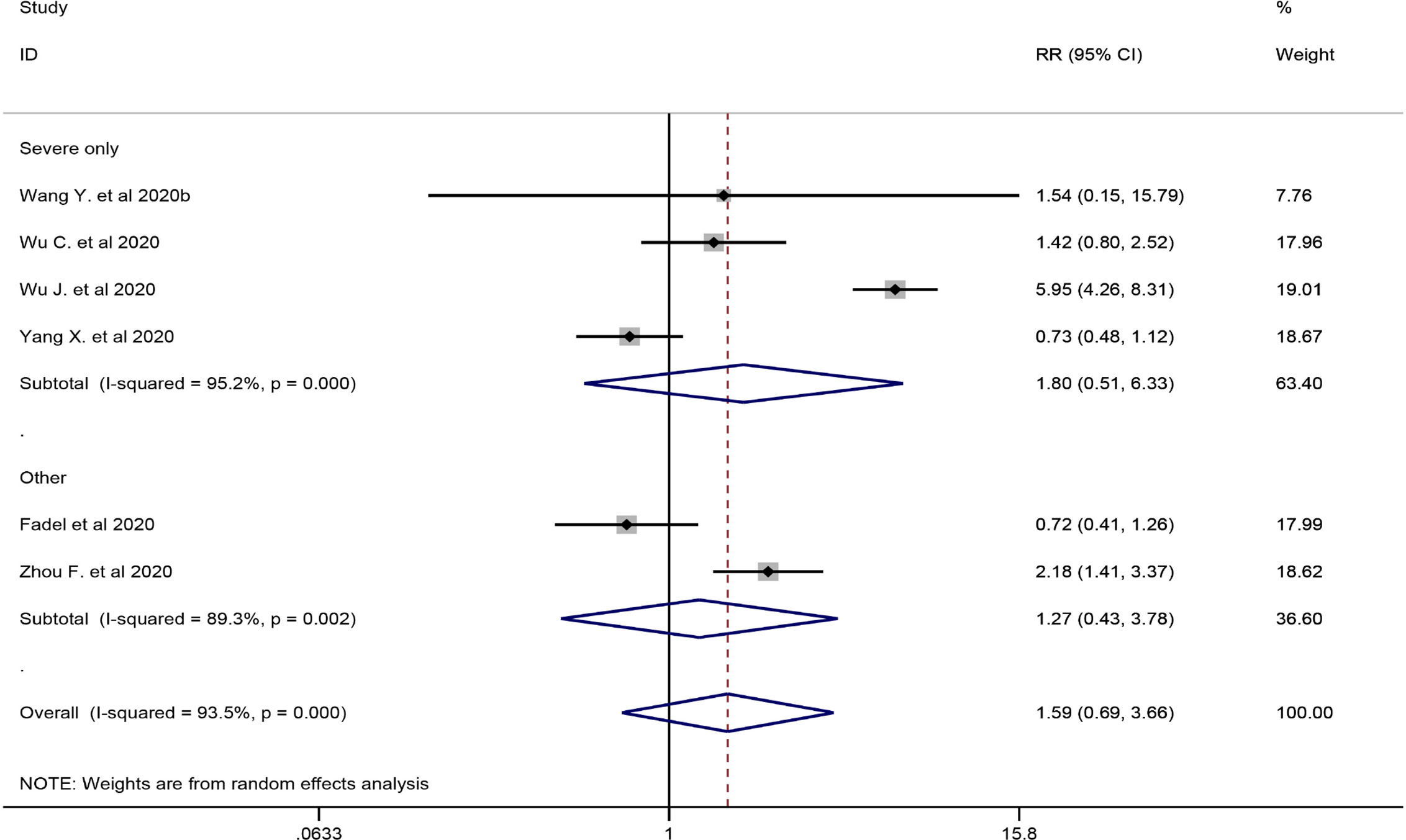
Six studies involving 2349 participants were included in the meta-analysis to explore the impact of corticosteroid treatment on mortality (Fadel et al., 2020; Wang et al., 2020b; Wu C. et al., 2020; Wu J. et al., 2020; Yang X. et al., 2020; Zhou F. et al., 2020). The results of the pooled analysis indicated that corticosteroids did not significantly reduce mortality (RR = 1.59, 95% CI [0.69, 3.66], I2 = 93.5%) (Figure 2). To address heterogeneity, we performed subgroup analysis. The results of the subgroup analysis showed no significant decrease in mortality in the severe only group (RR = 1.80, 95% CI [0.51, 6.33], I2 = 95.2%) and the other group (RR = 1.27, 95% CI [0.43, 3.78]) (Figure 2). The eligible cohort studies (corticosteroid group vs control group) that were not included in the quantitative synthesis demonstrated that corticosteroid therapy was not significantly associated with 28-day mortality (Lu et al., 2020).
Both included retrospective cohort studies (without control group) reported the outcome of mortality (Li and Liu, 2020; Zhou W. et al., 2020), and all patients involved in the two eligible studies had severe COVID-19. Zhou et al evaluated the efficacy of corticosteroid treatment in 15 Chinese patients with COVID-19 and reported that death had occurred in 46.7% of the population at the time of publication. Li et al. (2020) reported that the mortality of aggravation of COVID-19 within 48 h and after 48 h was 3.4% and 80%, respectively (Table 5). No deaths were reported in the included case studies; however, one study did not report mortality in patients using corticosteroids (Table 3) (Kui et al., 2020).
Virus Clearance Time
Four cohort studies including 247 subjects were included in the meta-analysis to explore the impact of corticosteroid treatment on virus clearance time (Fang et al., 2020a; Fang et al., 2020b; Ling et al., 2020; Ni et al., 2020; Zha et al., 2020). One out of the four studies compared the effects of corticosteroid treatment in the general group and severe group relative to the control group (Fang et al., 2020a; Fang et al., 2020b). Therefore, five trials were included in the quantitative synthesis. The pooled analysis data showed that the virus clearance time in the corticosteroid group was not shorter than that in the control group (WMD = 1.01, 95% CI [−0.91 to 2.92], I2 = 57%) (Figure 3). As described in the mortality section, we performed a subgroup analysis. The subgroup analysis showed that corticosteroid treatment did not notably reduce virus clearance time regardless of severe only (WMD = 0.85, 95% CI [−1.38 to 3.08], I2 = 0%) or other group (WMD = 1.43, 95% CI [−2.19 to 5.05], I2 = 77.8%) (Figure 3).
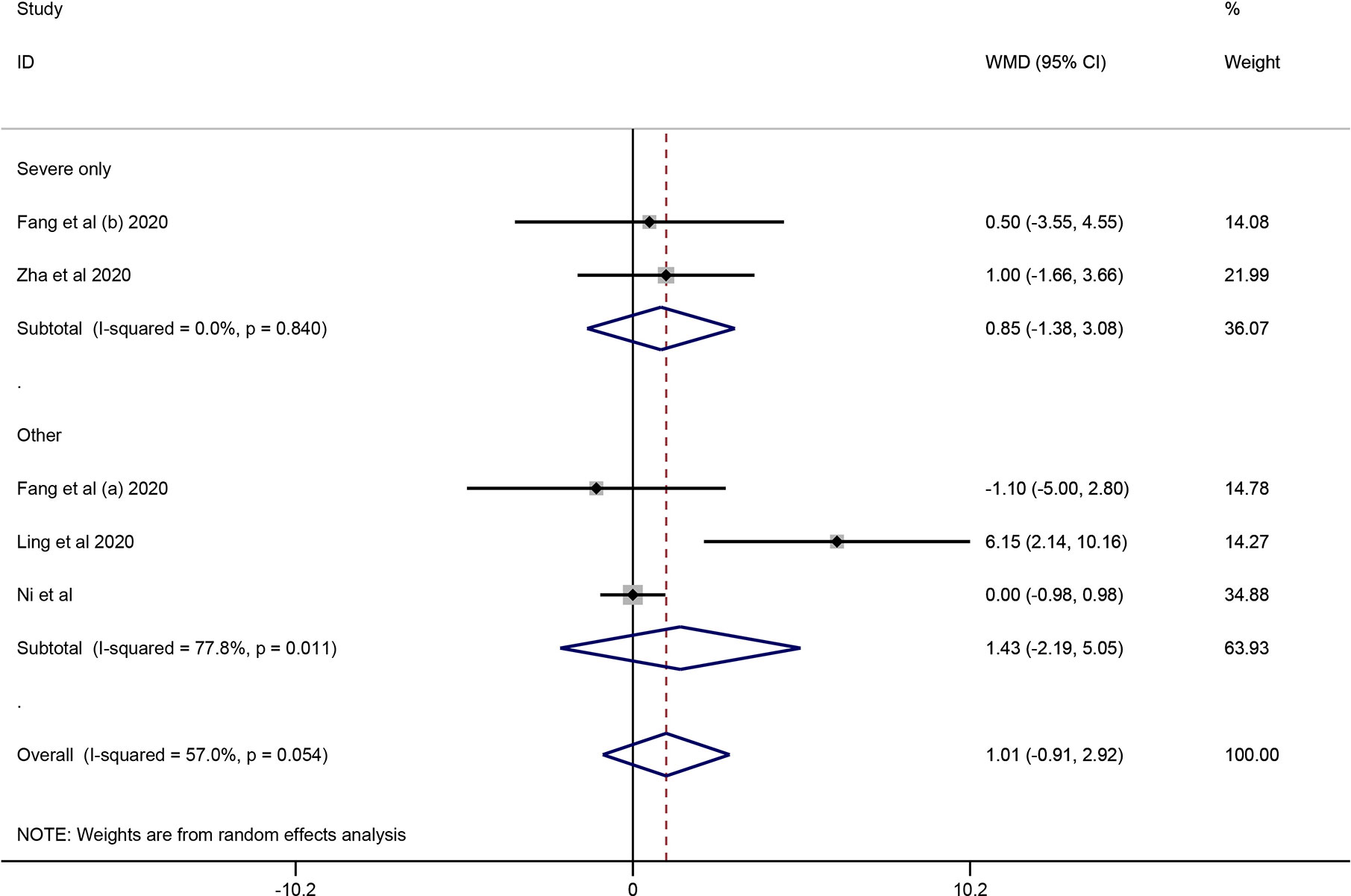
Figure 3 Forest plot of WMDs and 95% CIs for subgroup analysis of virus clearance time (severe only vs other).
All the included retrospective cohort studies (without control group) and case studies did not report virus clearance time in patients with COVID-19 after corticosteroid treatment.
Mechanical Ventilation
One cohort study involving 46 participants was included in the meta-analysis (Wang et al., 2020b). The results of the meta-analysis indicated that the use of mechanical ventilation was not different between the corticosteroid group and the control group (RR = 0.35, 95% CI [0.10, 1.18]) (Table 7). The use of mechanical ventilation in patients treated with corticosteroids was not clearly described in the included retrospective cohort studies (without control group). Among the included case studies, four reported that patients required mechanical ventilation (Chen et al., 2020; Coyle et al., 2020; Kui et al., 2020; Zhang et al., 2020), and two reported that patients did not require mechanical ventilation (Han et al., 2020; Luo et al., 2020). The remaining one study did not report the specific use of mechanical ventilation (Zheng et al., 2020).
Lengths of Hospitalization and ICU Hospitalization
Among the eligible studies, three studies involving 290 subjects were included in the meta-analysis of length of hospitalization (Fadel et al., 2020; Wang et al., 2020b; Zha et al., 2020), and one involving 46 participants was included in the meta-analysis of length of ICU hospitalization (Wang et al., 2020b). The pooled results showed that corticosteroid treatment was not associated with a shortened length of hospitalization (WMD = −3.17, 95% CI [−7.37, 1.04]), whereas it remarkably shortened the length of ICU hospitalization (WMD = −6.50, 95% CI [−7.63 to −5.37]) (Figure 4 and Table 7).
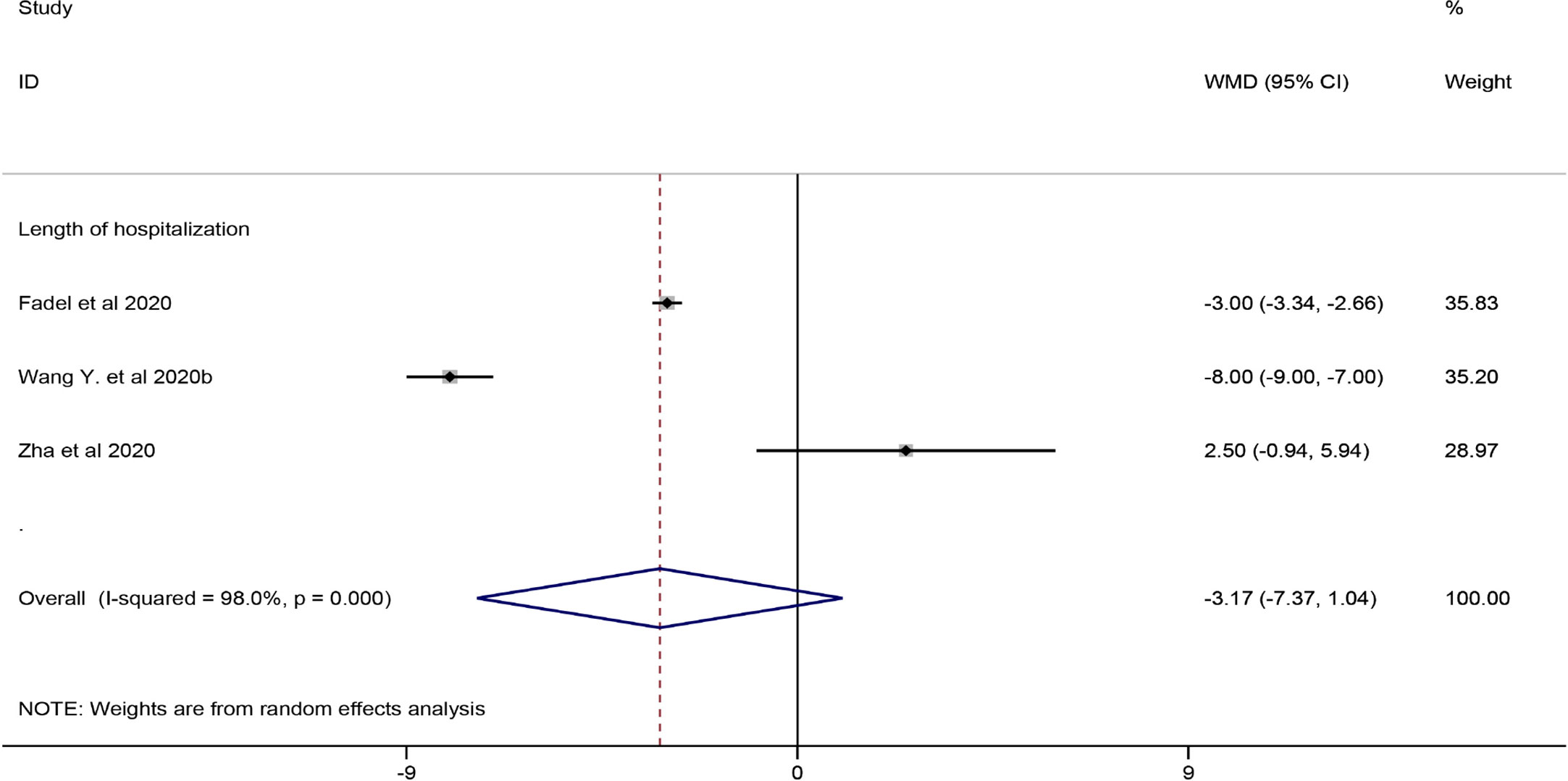
Figure 4 Forest plot of WMDs and 95% CIs for length of hospitalization hospitalization (corticosteroid group vs control group).
Safety
Only one included cohort study (corticosteroid group vs control group) reported AEs associated with corticosteroid therapy (Ni et al., 2020). The study reported that among the 51 patients treated with corticosteroids, 94.1% had transient hyperglycemia, 9.8% had hypokalemia, 9.8% had skin eruption, and 7.8% had increased blood pressure, but most of the AEs were mild and controllable. None of the included retrospective studies reported AEs in patients with COVID-19 after corticosteroid treatment.
Publication Bias
In this analysis, there was no publication bias on Egger test for mortality (p = 0.464) and virus clearance time (p = 0.174). The results of sensitive-analysis for mortality and virus clearance time see Supplementary Materials.
GRADE Assessment
The GRADE approach was used to evaluate the overall evidence. The quality of evidence is shown in Table 8. The quality of evidence of clinical improvement (recovery and duration of symptoms), VCT (severe only and other), mechanical ventilation and length of ICU hospitalization was very low, while the quality of evidence of mortality-severe only, mortality-other, and length of hospitalization was low.
Discussion
The main results of this systematic review and meta-analysis are as follows: (1) treatment with corticosteroid in COVID-19 patients did not significantly shorten the duration of symptoms but may promote clinical recovery; (2) Regardless of disease severity, corticosteroid therapy had no effect on mortality in patients with severe COVID-19; (3) corticosteroid therapy did not significantly reduce the virus clearance time in patients with COVID-19, irrespective of severity; (4) corticosteroid therapy in patients with COVID-19 did not affect the need for mechanical ventilation; (5) corticosteroid therapy was associated with a significantly decreased length of ICU hospitalization but had no impact on the length of hospitalization; and (6) corticosteroid therapy was associated with some mild and controllable AEs, including transient hyperglycemia, hypokalemia, skin eruption, and 7.8% had increased blood pressure.
Our results provide some evidence for the effectiveness of corticosteroids. As shown in Table 4, the meta-analysis indicated that compared with the control patients, the COVID-19 patients treated with corticosteroids showed a tendency of improved clinical recovery. This result was also supported by the retrospective cohort studies (without control group) and case studies we included. An eligible retrospective cohort studies (without control group) conducted by Li et al reported a high rate (96.55%) of clinical recovery of COVID-19 aggravation within 48 hours (Li and Liu, 2020). Additionally, except for one case study (Kui et al., 2020), all patients in the included case studies achieved clinical recovery. The above results indicated that treatment with corticosteroids in COVID-19 patients may increase the rate of clinical recovery. However, it is worth noting that the rate of clinical recovery of COVID-19 aggravation after 48 hours in Li et al’ study only reached 20%, which might indicate that clinical recovery was associated with corticosteroid administration time. Data from this study suggested that the use of corticosteroids did not reduce the risk of mortality. This is consistent with results of studies on MERS. A retrospective cohort study involving 309 subjects was conducted to investigate the association of corticosteroid therapy with mortality in critically ill patients with MERS and reported that there was no significant association with 90-day mortality (Arabi et al., 2017).
There has been a long debate about whether corticosteroid use might delay viral clearance in patients with viral pneumonia. The function of corticosteroids is to suppress the immune response as well as innate immunity. However, the elimination of the virus requires the involvement of innate immunity at the early stage, and the use of corticosteroids may delay the clearance of the virus (Ai et al., 2020; Isidori et al., 2020). Our results showed that corticosteroid therapy did not increase the virus clearance time regardless of the severity of COVID-19. Nevertheless, in the literature investigating MERS and influenza A (H7N9) viral pneumonia, the administration of corticosteroids was associated with delayed viral clearance (Cao et al., 2016; Arabi et al., 2017; Wang et al., 2020). Whether corticosteroid therapy delays viral clearance in patients with COVID-19 needs further study. Moreover, the utilization of mechanical ventilation was increased after corticosteroid treatment in patients with MERS and influenza (Arabi et al., 2017), while according to our results, corticosteroid therapy was not associated with the need for mechanical ventilation. Regarding the lengths of hospitalization and ICU hospitalization, our results revealed that corticosteroid treatment in patients with COVID-19 was not associated with a shorted length of hospitalization, whereas it remarkably shortened the length of ICU hospitalization. This might be related to the powerful pharmacological effects of the corticosteroid itself. A series of clinical studies demonstrated that low-dose or physiological-dose corticosteroid treatments in patients with septic shock caused by pulmonary infection significantly shortened the length of ICU hospital stay and reversed the progression of shock (Rhodes et al., 2017; Marik, 2018). Therefore, the clinical use of corticosteroids in patients with severe COVID-19 may reduce the length of ICU hospitalization.
In addition to focusing on the efficacy of corticosteroid therapy, its safety also needs to be fully evaluated. A retrospective analysis of corticosteroid therapy for ARDS caused by H1N1 showed that the early administration of corticosteroids resulted in an increased risk of mortality and increased incidence of acquired pneumonia (Brunbuisson et al., 2011). Some studies and reviews also showed that the adverse effects of corticosteroid therapy for coronavirus pneumonia were significantly greater than the therapeutic effects (Griffith et al., 2005; Brunbuisson et al., 2011; Ruan et al., 2014). Furthermore, a study on the treatment of SARS patients with corticosteroids demonstrated that the occurrence of adverse reactions was positively correlated with the dose and duration of corticosteroid use (Stockman et al., 2006). However, of all the literature included in this study, only one cohort study reported AEs of corticosteroid treatment, including transient hyperglycemia, hypokalemia, skin eruption, and increased blood pressure, in patients with COVID-19, and most of them were mild and controllable (Ni et al., 2020). Additional clinical studies are needed to explore the safety of corticosteroid treatment in patients with COVID-19 in the future.
Recently, emerging evidence from the initial clinical trial revealed a clear benefit of dexamethasone in patients with COVID-19 (Horby et al., 2020; Fadel et al., 2020). A large randomized, controlled, open-label (RECOVERY) trial conducted by Horby et al showed that the use of the dexamethasone to hospitalized COVID-19 patients enhanced the clinical outcome (Horby et al., 2020). In this study, 2104 patients were assigned to receive dexamethasone and 4,321 to receive usual care, and by the end, the incidence of death and duration of hospitalization in the dexamethasone group was lower and shorter than that in the usual care group. Similar results were observed in a recently published study that early treatment with methylprednisolone to hospitalized COVID-19 patients (Fadel et al., 2020), and a significant reduction in median hospital length of stay and mortality was observed in the early corticosteroid group. These beneficial effects might result from reducing the excessive inflammatory responses of COVID-19. As mentioned above, COVID-19 infection can causes a massive cytokine release (namely, cytokine storm), and leading to severe complications, such as acute respiratory distress syndrome (ARDS), disseminated thromboembolism, and hypotensive shock, and leads to a high risk of mortality (Isidori et al., 2020). These diseases are mostly driven by immunoinflammatory reactions. Dexamethasone, as a widely available and inexpensive corticosteroid, can attenuate the immune response and improved the clinical outcome in severe cases of COVID-19. However, the timing of corticosteroid use might be critical. COVID-19 can develop from mild to severe disease, characterized by an initial viral infection stage followed by lung inflammation, and then a hyper-inflammatory stage (Abdin et al., 2020; Siddiqi and Mehra, 2020). Early use of corticosteroids may delay the progression of the disease to the high inflammation stage (Fadel et al., 2020). This was consistent with a study included in our paper, which showed that the use of corticosteroid of COVID-19 aggravation after 48 hours significantly reduces the clinical recovery rate (Li et al., 2020). Moreover, the severity of the disease may also affect the outcome of corticosteroids. Some individuals with mild COVID-19 causes a specific adaptive immune response in the early stage (Isidori et al., 2020; Shi et al., 2020). The initial response begins with the recruitment of innate immune factors. Innate immunity at this stage is beneficial in eliminating the virus and preventing disease progression to severe stages (Shi et al., 2020). However, due to extensive immune suppression, the use of corticosteroids in mild patients with COVID-19 may delay the clearance of the virus by suppressing their own innate immunity (Isidori et al., 2020; Shi et al., 2020). Overall, the outcome of administering corticosteroids seems to largely depend on the severity of the disease and the timing of medication. The use of dexamethasone to achieve the optimal therapeutic improvement may be early use in severe patients with COVID-19.
There are some limitations in this study. First, only two included studies were conducted in America, and the remaining studies were conducted in China, and regional differences may impact the results of studies. Second, some outcomes, such as clinical recovery, duration of symptoms, mechanical ventilation and length of ICU hospitalization were measured in only a small number of studies or even just one study, leading to imprecision in the outcomes, thus decreasing the quality of the evidence. Third, considering the lack of literature and relevant data, we did not conduct subgroup analyses of the type and dosage of corticosteroids in this study.
In conclusion, our work complements recent systematic reviews of the efficacy and safety of corticosteroid treatment in patients with COVID-19 and enables, for the first time, quantitative synthesis of the effects of corticosteroid therapy considering cohort studies. The present study indicated that corticosteroid therapy was associated with clinical recovery and a significantly shortened length of ICU hospitalization, but it did not affect the mortality, the utilization of mechanical ventilation and the virus clearance time in COVID-19 patients. Furthermore, treatment with corticosteroids in patients with COVID-19 may cause mild adverse outcomes. The findings in our study do not demonstrate a strong efficacy of corticosteroid therapy, but it appears to improve prognosis and promote clinical recovery in patients with severe COVID-19.
Author Contributions
ZC and AX take responsibility for the integrity of data and accuracy of its analysis. WC and YL contributed to the conception and design of the study and writing of the manuscript. YL prepared the figures and tables. WC, LC, YC, SS, DX, and XC all contributed substantially to the literature search, data extraction and analysis, data interpretation and quality assessment.
Funding
This work was supported by National Key Research and Development Project (2020YFC2005700), Key Realm R&D Program of Guangdong Province (2019B030335001), and Medical Scientific Research Foundation of Guangdong Province of China (A2019407).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
We are grateful to Ying Guan for his help in confirming the validity of the statistical analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.571156/full#supplementary-material
References
Abdin, S. M., Elgendy, S. M., Alyammahi, S. K., Alhamad, D. W., Omar, H. A. (2020). Tackling the cytokine storm in COVID-19, challenges, and hopes. Life Sci. 257, 118054. doi: 10.1016/j.lfs.2020.118054
Ai, J., Li, Y., Zhou, X., Zhang, W. (2020). COVID-19: treating and managing severe cases. Cell Res. 30 (5), 370–371. doi: 10.1038/s41422-020-0329-2
Arabi, Y. M., Mandourah, Y., Alhameed, F., Sindi, A., Almekhlafi, G. A., Hussein, M. A., et al. (2017). Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respiratory Crit. Care Med. 197 (6), 757–767. doi: 10.1164/rccm.201706-1172OC
Baud, D., Qi, X., Nielsensaines, K., Musso, D., Pomar, L., Favre, G. (2020). Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 20 (7), 773. doi: 10.1016/S1473-3099(20)30195-X
Brunbuisson, C., Richard, J. M., Mercat, A., Thiebaut, A. C. M., Brochard, L. (2011). Early Corticosteroids in Severe Influenza A/H1N1 Pneumonia and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 183 (9), 1200–1206. doi: 10.1164/rccm.201101-0135OC
Cao, B., Gao, H., Zhou, B., Deng, X., Hu, C., Deng, C., et al. (2016). Adjuvant Corticosteroid Treatment in Adults With Influenza A (H7N9) Viral Pneumonia. Crit. Care Med. 44 (6), e318–e328. doi: 10.1097/CCM.0000000000001616
Chen, R., Tang, X., Tan, S., Liang, B., Wan, Z., Fang, J., et al. (2006). Treatment of Severe Acute Respiratory Syndrome With Glucosteroids: The Guangzhou Experience. Chest 129 (6), 1441–1452. doi: 10.1378/chest.129.6.1441
Chen, S., Yin, Q., Shi, H., Du, D., Chang, S., Ni, L., et al. (2020). A familial cluster, including a kidney transplant recipient, of Coronavirus Disease 2019 (COVID-19) in Wuhan, China. Am. J. Transplant. 20 (7), 1869–1874. doi: 10.1111/ajt.15903
Coyle, J., Igbinomwanhia, E., Sanchez-Nadales, A., Danciu, S., Chu, C., Shah, N. (2020). A Recovered Case of COVID-19 Myocarditis and ARDS Treated with Corticosteroids, Tocilizumab, and Experimental AT-001. JACC: Case Rep. 2 (9), 1331–1336. doi: 10.1016/j.jaccas.2020.04.025
Fadel, R., Morrison, A. R., Vahia, A., Smith, Z. R., Chaudhry, Z., Bhargava, P., et al. (2020). Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. Clin. Infect. Dis. ciaa601. doi: 10.1093/cid/ciaa601
Fang, X., Mei, Q., Yang, T., Li, L., Wang, Y., Tong, F., et al. (2020a). Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J. Infect. 81 (1), 147–178. doi: 10.1016/j.jinf.2020.03.039
Fang, X., Mei, Q., Yang, T., Li, L., Wang, Y., Tong, F., et al. (2020b). Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J. Infect. 81 (1), 147–178. doi: 10.1016/j.jinf.2020.03.039
General Office of National Health Commission of People’s Republic of China, O. o. N. A. o. T. C. M (2020). Diagnosis and Treatment Protocol for COVID-19 (Trial Seventh Edition). China Med. 15 (6), 801–805. doi: 10.3760/j.issn.163477.2020.06.001
Griffith, J. F., Antonio, G. E., Kumta, S. M., Hui, D. S. C., Wong, J. K. T., Joynt, G. M., et al. (2005). Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology 235 (1), 168–175. doi: 10.1148/radiol.2351040100
Han, Y., Jiang, M., Xia, D., He, L., Lv, X., Liao, X., et al. (2020). COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin. Immunol. 214, 108413–108413. doi: 10.1016/j.clim.2020.108413
Horby, P., Lim, W. S., Emberson, J., Mafham, M., Bell, J. L., Linsell, L., et al. (2020). Effect of dexamethasone in Hospitalized Patients with COVID -19: Preliminary Report. N Engl. J. Med. 58 (9), 133. doi: 10.1056/NEJMoa2021436
Hozo, S. P., Djulbegovic, B., Hozo, I. (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Method. 5 (1), 13–13. doi: 10.1186/1471-2288-5-13
Isidori, A. M., Arnaldi, G., Boscaro, M., Falorni, A., Giordano, C., Giordano, R., et al. (2020). COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J. Endocrinol. Invest. 43 (8), 1141–1147. doi: 10.1007/s40618-020-01266-w
Kui, L., Fang, Y., Deng, Y., Liu, W., Wang, M., Ma, J., et al. (2020). Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. (Engl.) 133 (9), 1025–1031. doi: 10.1097/CM9.0000000000000744
Li, W., Liu, D. (2020). Effective window stage of methylprednisolone sodium succinate on the treatment of COVID⁃19. Med. J. Wuhan Univ., 1–5. doi: 10.14188/j.1671⁃8852.2020.0168
Li, H., Chen, C., Hu, F., Wang, J., Zhao, Q., Gale, R., et al. (2020). Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia 34 (6), 1503–1511. doi: 10.1038/s41375-020-0848-3
Ling, Y., Xu, S., Lin, Y., Tian, D., Zhu, Z., Dai, F., et al. (2020). Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 133 (9), 1039–1043. doi: 10.1097/CM9.0000000000000774
Lu, X., Chen, T., Wang, Y., Wang, J., Yan, F. (2020). Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit. Care 24 (1), 241. doi: 10.1186/s13054-020-02964-w
Luo, W., Wei, M., Wei, H. L., Li, J., Yi, Q. (2020). A case of severe COVID-19 with glucocorticoid-induced diabetes and a preliminary study on the use of glucocorticoids. Chin. J. Respiratory Crit. Care Med. 19 (02), 176–180. doi: 10.7507/1671-6205.202002016
Marik, P. E. (2018). Steroids for sepsis: yes, no or maybe. J. Thorac. Dis. 10 (Suppl 9), S1070–S1073. doi: 10.21037/jtd.2018.04.35
Meader, N., King, K., Llewellyn, A., Norman, G., Brown, J., Rodgers, M., et al. (2014). A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst. Rev. 3 (82). doi: 10.1186/2046-4053-3-82
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 (1):1. doi: 10.1186/2046-4053-4-1
Moreno, G., Rodríguez, A., Reyes, L. F., Gomez, J., Sole-Violan, J., Díaz, E., et al. (2018). Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 44 (9), 1470–1482. doi: 10.1007/s00134-018-5332-4
Ni, Q., Ding, C., Li, Y., Zhao, H., Liu, J., Zhang, X., et al. (2020). Effect of low-to-moderate dose glucocorticoids on viral clearance in COVID-19: a retrospective study. Chin. J. Clin. Infect. Dis. 13 (1), 21–24. doi: 10.3760/cma.j.issn.1674-2397.200.01.005
Organization, W. H. (2020). Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected (Geneva: World Health Organization).
Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. M., Antonelli, M., Ferrer, R., et al. (2017). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 43 (3), 304–377. doi: 10.1007/s00134-017-4683-6
Ruan, S., Lin, H., Huang, C., Kuo, P., Wu, H., Yu, C. (2014). Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Crit. Care 18 (2), R63. doi: 10.1186/cc13819
Russell, C. D., Millar, J. E., Baillie, J. K. (2020). Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395 (10223), 473–475. doi: 10.1016/S0140-6736(20)30317-2
Sehgal, V. N., Malhotra, R. (2019). Pharmacology and Therapeutics of Corticosteroids Sparing Maintenance Immunosuppressive/Adjunct Therapy Drugs. Skinmed 17 (3), 172–179. eCollection 2019.
Shi, Y., Wang, Y., Shao, C., Huang, J., Gan, J., Huang, X., et al. (2020). COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 27 (5), 1451–1454. doi: 10.1038/s41418-020-0530-3
Siddiqi, H. K., Mehra, M. R. (2020). COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Heart Lung Transplant. 39 (5), 405–407. doi: 10.1016/j.healun.2020.03.012
Stockman, L. J., Bellamy, R., Garner, P. (2006). SARS: Systematic review of treatment effects. PloS Med. 3 (9), e343. doi: 10.1371/journal.pmed.0030343
Veronese, N., Demurtas, J., Yang, L., Tonelli, R., Barbagallo, M., Lopalco, P., et al. (2020). Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature. Front. Med. (Lausanne) 7, 170. doi: 10.3389/fmed.2020.00170
Wang, C., Horby, P., Hayden, F. G., Gao, G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet 395 (10223), 470–473. doi: 10.1016/S0140-6736(20)30185-9
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323 (11), 1061–10695. doi: 10.1001/jama.2020.1585
Wang, Y., Jiang, W., He, Q., Wang, C., Wang, B., Zhou, P., et al. (2020a). Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv. doi: 10.1101/2020.03.06.20032342
Wang, Y., Jiang, W., He, Q., Wang, C., Wang, B., Zhou, P., et al. (2020b). A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct. Target Ther. 5 (1), 57. doi: 10.1038/s41392-020-0158-2
Wells, G. A., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2014). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Psychology.
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., et al. (2020). Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 180 (7), 1–11. doi: 10.1001/jamainternmed.2020.0994
Wu, J., Huang, J., Zhu, G., Liu, Y., Xiao, H., Zhou, Q., et al. (2020). Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: a retrospective cohort study. medRxiv. doi: 10.1101/2020.05.11.20097709 preprint.
Yang, J., Yang, L., Luo, R., Xu, J. (2020). Corticosteroid administration for viral pneumonia: COVID-19 and beyond [published online ahead of print 2020 Jun 27]. Clin. Microbiol. Infect. S1198-743X (20), 30364–30365. doi: 10.1016/j.cmi.2020.06.020
Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J., Liu, H., et al. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8 (5), 475–481. doi: 10.1016/S2213-2600(20)30079-5
Zha, L., Li, S., Pan, L., Tefsen, B., Li, Y., French, N., et al. (2020). Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med. J. Aust. 212 (9), 416–420. doi: 10.5694/mja2.50577
Zhang, S., Li, D., Chen, H. Z., Zheng, D., Zhou, Y., Chen, B., et al. (2020). Dynamic inflammatory response in a critically ill COVID-19 patient treated with corticosteroids. J. Zhejiang Univ. (Med. Sci.) 49 (02), 220–226. doi: 10.3785/j.issn.1008-9292.2020.03.10
Zhao, Z., Zhang, F., Xu, M., Huang, K., Zhong, W., Cai, W., et al. (2003). Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microbiol. 52 (Pt 8), 715–720. doi: 10.1099/jmm.0.05320-0
Zheng, K., Zhang, Y., Wang, Q., Xu, Y., Wang, H., Kong, X., et al. (2020). Severe coronavirus disease 2019 successfully treated with glucocorticoid and high-dose intravenous immunoglobulin: a case report and analysis of clinical experience. Acad. J. Second Military Med. Univ. 41 (02), 181–185. doi: 10.16781/j.0258-879x.2020.02.0181
Zhong, N. S., Zeng, G. Q. (2003). Our Strategies for Fighting Severe Acute Respiratory Syndrome (SARS). Am. J. Respiratory Crit. Care Med. 168 (1), 7–9. doi: 10.1164/rccm.200305-707OE
Zhong, H., Wang, Y., Zhang, Z., Liu, Y., Le, K., Cui, M., et al. (2020). Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol. Res. 157, 104872. doi: 10.1016/j.phrs.2020.104872
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (10229), 1054–1062. doi: 10.1016/S0140-6736(20)30566-3
Keywords: corticosteroid, COVID-19, SARS-CoV-2, efficacy, meta-analysis
Citation: Cheng W, Li Y, Cui L, Chen Y, Shan S, Xiao D, Chen X, Chen Z and Xu A (2020) Efficacy and Safety of Corticosteroid Treatment in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 11:571156. doi: 10.3389/fphar.2020.571156
Received: 10 June 2020; Accepted: 17 August 2020;
Published: 09 September 2020.
Edited by:
Per-Johan Jakobsson, Karolinska Institutet (KI), SwedenReviewed by:
Simona Gabriela Bungau, University of Oradea, RomaniaArpan Acharya, University of Nebraska Medical Center, United States
Copyright © 2020 Cheng, Li, Cui, Chen, Shan, Xiao, Chen, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuoming Chen, tchzm@21cn.com; Anding Xu, tlil@jnu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Wenwen Cheng
Wenwen Cheng Yufeng Li2†
Yufeng Li2† Xiaoyun Chen
Xiaoyun Chen