Abstract
Background
Collaborative (“shared-care”) models of postoperative care improve outcomes in patients undergoing surgery for hip fracture. Despite being widely adopted, it is unclear if similar benefits of shared-care models exist for other at-risk surgical patient populations. Thus, we performed a systematic review to understand the impact of shared-care models.
Methods
EMBASE, MEDLINE, CINAHL, and Cochrane Central Register databases were searched for prospective studies examining an in-hospital shared-care approach to postoperative management of adult non-cardiac surgery patients. The primary outcome was a composite of in-hospital mortality and mortality of up to 30 days. Secondary outcomes were long-term mortality (> 90 days) and hospital length of stay. Tertiary outcomes included quality of life and health utility measures. Risk of bias was assessed using Cochrane Collaboration tools.
Results
Six thousand eight hundred and ninety-six citations were reviewed and four studies (n = 987 patients) met the inclusion criteria—two randomized-controlled trials (RCT, n = 729 patients) and two non-randomized-controlled trials (NRCT, n = 258 patients). All studies were conducted in the elective surgical setting. There was no association between shared-care and control groups for in-hospital mortality (Peto odds ratio, 1.76; 95% confidence interval [CI], 0.65 to 4.80), or hospital length of stay (mean difference, −1.41; 95% CI, −3.18 to 0.35). Reporting of other outcomes was limited. Both RCTs were judged to be at high risk of bias for blinding and both NRCTs were judged to be at moderate risk of bias for reported outcomes.
Conclusion
Overall, there was limited high-quality evidence to evaluate the effect of postoperative shared-care. Well-designed interventional studies, perhaps targeting higher risk surgical populations, are needed.
Registration
PROSPERO (CRD42018094943); registered 16 May, 2018.
Résumé
Contexte
Les modèles de soins postopératoires collaboratifs (« soins partagés ») améliorent le pronostic des patients subissant une chirurgie pour fracture de la hanche. Malgré l’adoption répandue de ce modèle de soins partagés, nous ne savons pas s’ils offrent des avantages semblables pour d’autres populations chirurgicales de patients à risque. Nous avons par conséquent réalisé une revue systématique afin de comprendre l’impact des modèles de soins partagés.
Méthode
Les bases de données EMBASE, MEDLINE, CINAHL et Cochrane Central Register ont été examinées afin d’en extraire les études prospectives examinant l’utilisation de soins partagés en milieu hospitalier pour la prise en charge postopératoire de patients chirurgicaux adultes hors chirurgie cardiaque. Le critère d’évaluation principal était un composé de la mortalité hospitalière et de la mortalité jusqu’à 30 jours. Les critères d’évaluation secondaires étaient la mortalité à long terme (> 90 jours) et la durée de séjour hospitalier. Les critères tertiaires comprenaient des mesures de la qualité de vie et de la santé. Le risque de biais a été évalué à l’aide d’outils du Cochrane Collaboration.
Résultats
Six mille huit cent quatre-vingt-seize citations ont été passées en revue et quatre études (n = 987 patients) ont répondu à nos critères d’inclusion, soit deux études randomisées contrôlées (ERC, n = 729 patients) et deux études non randomisées contrôlées (ENRC, n = 258 patients). Toutes les études ont été réalisées dans un contexte de chirurgie non urgente. Aucune association entre les soins partagés et les groupes témoin n’a été observée en ce qui touchait à la mortalité hospitalière (rapport de cotes de Peto, 1,76; intervalle de confiance [IC] 95 %, 0,65 à 4,80) ou à la durée de séjour hospitalier (différence moyenne, −1,41; IC 95 %, −3,18 à 0,35). La communication de nos autres critères d’évaluation était limitée. Il a été estimé que les deux ERC affichaient un risque élevé de biais de non-respect de l’insu et les deux ENRC affichaient un risque modéré de biais dans la communication des résultats.
Conclusion
Globalement, les données probantes de qualité élevée étaient limitées pour évaluer l’effet de soins postopératoires partagés. Des études d’intervention bien conçues, ciblant peut-être des populations chirurgicales à risque plus élevé, sont nécessaires.
Enregistrement de l’étude
PROSPERO (CRD42018094943); enregistrée le 16 mai 2018.
Similar content being viewed by others
More than 200 million adults undergo surgery annually and more than 1 million die within 30 days.1,2 In particular, major perioperative complications account for at least 70% of perioperative mortality,3 and are associated with subsequent complications, longer hospital stay, and increased medical expenses.3,4,5,6,7
Providing safe and effective postoperative care is an integral part of surgical care. Surgeons are can be busy in operating rooms, which may limit their ability to rapidly respond to medical complications on surgical floors. One study demonstrated that the median duration of clinically important intraoperative hypotension was 15 min, whereas on the first postoperative day it was 150 min.8 This suggests a need for pathways to facilitate timely identification and management of postoperative adverse events. Collaborative (“shared-care”) models of postoperative care that involve clinicians from more than one speciality may improve surveillance of at-risk patients and also increase clinician availability. A systematic review of shared-care models demonstrated an improvement in mortality and length of stay in older people undergoing urgent hip fracture surgery when co-managed by both surgeons and geriatricians compared with surgeons alone.9 Aside from this highly select population (i.e., hip fracture patients), it is unclear whether patients undergoing other types of non-cardiac surgery could benefit from shared-care management.
This systematic review and meta-analysis was performed to evaluate whether postoperative shared-care models led by physicians or nurses improve outcomes for adult patients undergoing elective or emergent inpatient non-cardiac surgeries. Specifically, we looked to synthesize the effect of shared-care on mortality, hospital length of stay, quality of life, and healthcare utilization, compared with non-shared-care models from prospectively designed studies. The results of this systematic review may help inform clinical guidelines for non-cardiac surgery patients,10 and identify high priority knowledge gaps.
Methods
The protocol for this review was prospectively registered with the PROSPERO database of systematic reviews (CRD42018094943) on 16 May, 2018. The review was carried out in accordance with methodologic recommendations from the Cochrane Collaboration, and the final report was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Electronic Supplementary Material [ESM] eAppendix 1).11
Eligibility criteria
Population
Adults (≥ 18 yr of age) having either elective or emergent inpatient non-cardiac surgery were included. In the case of studies including mixed populations or procedures, if at least 50% of the population met inclusion criteria, the study was included. Otherwise, data were included from mixed studies only if relevant patient level or subgroup-specific data were available. Non-cardiac vascular procedures were included (e.g., aortic abdominal aneurysm repair, femoral/bypass surgery). Cardiac-specific procedures were excluded (e.g., coronary artery bypass grafting), as these patients are routinely cared for postoperatively in intensive care units. Patients who had hip fracture surgery were excluded given the previously published systematic review focused on this patient population.9 Studies of patients undergoing elective hip replacements were eligible. Outpatient surgeries and invasive procedures (e.g., colonoscopies and ophthalmologic procedures such as cataract surgery) were excluded.
Intervention
Studies of an in-hospital collaborative approach to postoperative management were included. This included postoperative care involving surgeons, anesthesiologists, or internal medicine specialists (i.e., cardiologist, geriatrician, general internist) and nurses. If the intervention was restricted to preoperative, rehabilitative, or post-discharge care, then these studies were excluded. Postoperative care provided in intermediate care units (e.g., “step-down”) or intensive care units were excluded because we viewed this type of care and acute monitoring as substantially different from the typical ward-based care provided to the majority of elective surgical patients. The effect of surgical step-down units has been evaluated in a separate systematic review.12
Comparators
Studies must have included a prospectively collected control group that received standard (i.e., non-shared) postoperative care management.
Outcomes
The Institute for Healthcare Improvement Triple Aim was used as a framework (i.e., improved health, lower costs, and improved patient experience).13 The primary outcome was in-hospital mortality or mortality up to 30 days. Secondary outcomes were long-term mortality defined as mortality at 90 days or more after the surgical procedure and hospital length of stay defined as the number of days from hospital admission to discharge. Tertiary outcomes included functional or health-related quality of life and health utility measures assessed at any time point during the study.
Study design
Interventional studies with a comparator-control were included such as randomized-controlled trials (RCTs), cluster RCTs, and non-randomized-controlled trials (NRCTs). Non-randomized-controlled trials included were interrupted time series and controlled before-and-after studies that included a prospectively collected control group. Before-and-after studies with a historic cohort control group and other types of historical observational studies (i.e., cohort studies, case-control studies, case series, and cross-sectional studies) were excluded. Full-text articles in any language were considered and there was no restriction on year of publication. Unpublished grey literature, abstracts, conference abstracts, commentaries, letters, reviews, and editorials were excluded as methodologic descriptions would be insufficient to assess potential eligibility and their risk of bias.
Information sources
MEDLINE (OVID interface, including In-Process and e-Pub Ahead of Print), EMBASE (OVID interface), Cumulative Index to Nursing and Allied Health Literature (CINAHL) database, and the Cochrane Central Register of Controlled Trials (Wiley interface) were each searched from inception to February 2018. Clinical trial registries were searched to identify ongoing and completed trials. Specifically, Clinicaltrials.gov and the International Prospective Register of Systematic Reviews (PROSPERO) were searched to identify ongoing or recently completed trials or systematic reviews. To further ensure a comprehensive systematic literature search, we examined reference lists of included studies and relevant reviews identified through the search. Finally, we circulated a bibliography of included articles to the systematic review team for feedback.
Search strategy
A specific search strategy was created in collaboration with a health sciences librarian with expertise in the design of systematic literature searches. The literature strategies were developed using keywords related to shared-care and non-cardiac surgery. The syntax and medical subject headings used in the EMBASE search strategy were modified for other databases used in this systematic review, which included PubMed/Medline, Cochrane Central Register of Controlled Trials, and Cumulative Index to Nursing and Allied Health Literature (CINAHL). There were no date restrictions. The search strategies are included in ESM eAppendix 2. A second librarian performed a Peer Review of Electronic Search Strategies (PRESS)14 (ESM eAppendix 3).
Data management
The literature search results were uploaded to Distiller Systematic Review Software (DistillerSR®, Evidence Partners, Ottawa, ON, Canada). Distiller is an audit-ready, cloud-based software program that allows for transparent and reproducible work required for an accurate review.
Selection process
Reviewers (S.M., M.M.L., J.M., D.Y.) independently screened the titles and abstracts from the search results using the predefined inclusion criteria. If a reviewer thought that the title or abstract could fulfill eligibility criteria then the full-text study would be assessed for eligibility. A calibration exercise was performed on the first ten studies to refine the screening question before formally commencing the screening process. Two review authors (S.M., J.M.) independently assessed the eligibility of the full-text articles. Discrepancies between the reviewers were resolved by discussion or with a third-party member (M.M.L.) if a consensus could not be established. Reasons for excluding studies were recorded. Ottawa Hospital employees with fluency in the non-English article languages were contacted for assistance to determine article eligibility.
Data collection process
Standardized drafts of data extraction forms were designed to collect all data items of interest from the included studies. The drafts of the data extraction were used to inform the development of the online abstraction tool using DistillerSR. A calibration exercise was conducted by two independent reviewers (S.M., J.M.) before formally beginning data extraction. Data were extracted independently by two reviewers (S.M., J.M.). Disagreements between reviewers were resolved by discussion or with a third-party member (M.M.L.) if a consensus could not be reached.
Data items
We collected information related to study and patient characteristics (year of publication, authors, country, trial design, sample size, age, gender, body mass index, functional/physical status, surgical service, surgical procedure, surgical setting, and follow-up), intervention characteristics (team composition, surgical service, timing, and frequency of follow-up), and outcome data (in-hospital mortality, long-term mortality, hospital length of stay, quality of life, and health utility measures). Because the number of included studies was small, each investigation was not categorized by the type of care model as described in the protocol; instead a full description of the care model was reported.
Risk of bias assessment
Randomized-controlled trials that met inclusion criteria were assessed for risk of bias using the Cochrane Risk of Bias tool for RCTs.15 Non-randomized-controlled trials were assessed for risk of bias using the Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool.16 Risk of bias was assessed by two independent reviewers (S.M., J.M.). Disagreements were resolved first by discussion or by consulting a third-party member (M.M.L.) for arbitration.
Statistical analysis
Studies were pooled using Comprehensive Meta-Analysis (CMA) software (CMA version 3, Biostat, NJ, USA). For the primary outcome (in-hospital mortality), fixed-effects Peto odds ratios (ORs) were presented with accompanying 95% confidence intervals (CIs). Peto ORs were used because of the expected rarity of events. This method allowed for the inclusion of continuity corrections of 0.5 for all zero cells, allowing us to estimate ORs for studies reporting no events.17 For hospital length of stay, a mean difference was calculated using a random effects inverse variance model and presented with accompanying 95% CIs. Heterogeneity of effect sizes in the pooled proportions was assessed among included studies using the I2 statistic.18 The thresholds for interpretation of I2 were as follows: 0-40% might not be important, 30-60% may represent moderate heterogeneity, 50-90% may represent substantial heterogeneity, and 75-100% is considerable heterogeneity.18 Pre-specified sensitivity and subgroup analyses were not performed because insufficient data were available.
Confidence in cumulative evidence
The quality of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.19 The quality of evidence was assessed by two independent reviewers (S.M., J.M.) across the domains of risk of bias, consistency, directness, and precision. Quality was assigned as one of four GRADE scores (0-4) reflecting high, moderate, low, or very low quality evidence.19
Results
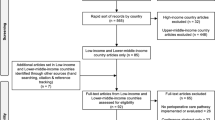
The literature search identified 6,896 unique citations following the removal of duplicates. Screening citations by title and then abstract identified 59 potentially relevant studies for full-text review. Based on full-text review, four studies fulfilled eligibility criteria and were included in this systematic review. One study consisted of two publications for short-term20 and long-term outcomes.21 PRISMA diagram and reasons for full-text study exclusion are presented in Fig. 1.
Study characteristics
The four studies included 987 patients. The studies by Huddleston et al.22 and Harari et al.23 evaluated shared-care in the orthopedic setting (n = 577 patients), Chen et al.24 in the bariatric surgery setting (n = 150),24 and a study by Hempenius et al.20 included mixed surgical settings (n = 260) (Table 1). Patients in all studies underwent elective surgery. Studies by Huddleston et al.22 and Hempenius et al.20 were RCTs (n = 729 patients) and studies by Harari et al.23 and Chen et al.24 were NRCTs (n = 258 patients). Studies followed patients for 30 days,23 three months,20 or 12 months,24 and one study did not report the duration of follow-up.22 Primary outcomes reported were hospital length of stay22,23 and inpatient postoperative medical complication rate.22 Huddleston et al.22 reported secondary outcomes of patient satisfaction with overall care, provider satisfaction regarding the shared-care model, and direct costs of hospital care.22 Hempenius et al.20 reported the incidence of delirium up to ten days postoperatively as a primary outcome and reported delirium severity, length of hospital stay, mortality, complications, care dependency, quality of life, return to independent preoperative living situation, and additional care at home as secondary outcomes.
Intervention characteristics
Intervention characteristics are presented in Table 2. Huddleston et al.22 included a hospitalist-orthopedic surgical team that consisted of general internal medicine hospitalists, an orthopedic surgical team, and surgical nurses. Care by this team was compared with care by a surgical team. Harari et al.23 investigated proactive care of older people undergoing surgery consisting of postoperative care by a consultant geriatrician, nurse specialist, occupational therapist, physiotherapist, and social worker compared with a cohort where care was provided by the surgical team alone. Chen et al.24 included postoperative care by a bariatric team, which consisted of a bariatric surgeon, case manager, bariatric nurse, dietician, anesthetist, endoscopist, and radiologist compared with care by a surgeon alone. Hempenius et al.20 included a geriatric team consisting of a geriatrician and geriatric nurse. Care by this team was compared with care by a surgical team where a geriatrician provided additional care at the request of the treating physician.
Reported outcomes
Primary outcome
Studies by Huddleston et al.,22 Harari et al.,23 and Hempenius et al.20 (n = 837 patients) reported in-hospital or mortality within 30 days. There was no significant difference in the risk of mortality between the shared-care (n = 10/413) and control groups (n = 6/424) (Peto OR, 1.76; 95% CI, 0.65 to 4.80; I2 = 47%) (Fig. 2A). There was insufficient data to explore this outcome in our planned subgroup analysis.
Secondary outcomes
Four studies (n = 987 patients) reported hospital length of stay20,22,23,24 however, there was no significant difference between shared-care (n = 513) and control (n = 474) groups (mean difference, −1.41 days; 95% CI, −3.18 to 0.35; I2 = 87%; Fig. 2B). Hempenius et al.20 reported that the percentage of patients who stayed in hospital longer than eight days did not differ between the two groups (50% vs 43%, respectively; OR, 1.28; 95% CI, 0.77 to 2.12). No studies reported on long-term mortality (≥ 90 days).
Tertiary outcomes
One RCT reported on function and health-related quality of life,20 including a three-month post-hoc analysis.21 There was no significant improvement in care dependency at discharge (OR, 0.93; 95% CI, 0.52 to 1.65)20 or at the three-month follow-up (OR, 1.19; 95% CI, 0.70 to 2.02).21 One study assessed health-related quality of life using the Short-Form 36 (SF-36).25 There was no significant increase in physical function at discharge (OR, 1.02; 95% CI, 0.56 to 1.87)20 or at the three-month follow-up (OR, 1.33; 95% CI, 0.77 to 2.30).21 There was also no significant increase in mental health at discharge (OR, 0.80; 95% CI, 0.47 to 1.34)20 or at the three-month follow-up (OR, 0.84; 95% CI, 0.50 to1.42).21 No studies reported on health utility measures.
Risk of bias assessment
In the two RCTs, risk of bias was judged as high for the domain of blinding of participants and personnel, because of the inability to blind. The remaining domains were considered to be at low risk or unclear (Fig. 3). For both NRCTs,23,24 the outcomes assessed were both judged to be at moderate risk of bias (Table 3).
Strength of evidence
The GRADE evidence profile is presented in ESM eAppendix 4. We found the strength of evidence for our outcomes to be of low quality.
Discussion
This systematic review evaluated the impact of postoperative shared-care models on mortality and other outcomes in adults having inpatient non-cardiac surgery, excluding hip fractures. Although the available evidence is limited in quality and quantity, there is currently no strong indication that shared-care models improve rates of in-hospital mortality or hospital length of stay. There was limited data on other patient-important outcomes, such as function, quality of life, and long-term survival. Whereas strong systematic review evidence that shared-care models substantially improve outcomes in hip fracture surgery already exists,9 our findings suggest that either the effect of shared-care is unique to the high-risk geriatric population having hip fracture surgery, or more likely, that non-hip fracture shared-care models have been inadequately evaluated in the current literature. In fact, in a systematic review of the hip fracture population,9 a 40% relative reduction in the risk of in-hospital death was observed. Given the baseline mortality rate in our control group (1.4%), we would require over 5,000 patients per group to detect a similar difference.
Given the strong association between shared-care models and decreased mortality previously described (where eight of nine studies found shared-care to be directionally associated with lower mortality rates), we must consider why the current study did not find similar evidence. This may be due to the small number of studies that we were able to identify; however, it may also relate to the patient populations and surgical procedures that were studied. The overall low event rate for in-hospital mortality in our review is reflective of the low-risk populations and procedures studied and is substantially lower than rates typically reported after major elective non-cardiac surgery. Furthermore, short-term mortality after hip fracture surgery typically exceeds 5%.9 This may suggest that shared-care management could be more beneficial for high-risk patient populations; however, we were unable to identify studies that had investigated these types of populations. One study also included a variety of procedures that were categorized as major, intermediate, and minor depending on the duration of the procedure and tumour localization.20 Nevertheless, due to the small number of studies and events included in our review, we could not investigate the impact of shared-care on the different surgical procedures and risk profiles. Findings of our review of surgical step-down units were similar in that few studies had been conducted and the limited available evidence revealed few differences in outcomes.12
It is difficult to determine the impact that biases may have had on specific outcomes in this review. Different types of surgical procedures were evaluated among the four studies that assessed length of stay. Each procedure is associated with its own inherent risk, some higher than others, which may have contributed to the heterogeneity found. Huddleston et al.22 was judged to be at low risk of bias for the majority of domains while Harari et al.23 was judged to be at moderate risk. While these studies were similar in terms of the surgical setting, it is difficult to determine the impact that biases may have had on specific outcomes in this review. Through a detailed GRADE assessment of our outcomes we also found that the strength of evidence was low across all outcomes.
Our systematic review was able to identify important knowledge gaps. First, postoperative shared-care management after non-cardiac surgery has only been evaluated in highly selective patient populations. Future studies could be conducted in other settings, perhaps with higher risk patients, to adequately evaluate if there is a benefit of shared-care management. Second, our systematic review also identified that quality of life and health utility measures have yet to be evaluated in this context.
Our systematic review has several limitations. First, there were a limited number of interventional studies included. These studies had small sample sizes, which could have potentially biased the association toward the null hypothesis. Second, two studies also reported zero events for our primary outcome, making an accurate estimation of the odds ratio difficult. To be conservative in our estimate, a correction factor of 0.5 was applied to each zero cell;26 however, there is no agreed upon standard approach for this analytical issue.27,28 Third, both RCTs had an unclear risk of bias for four domains as they did not report some methodologic details. Both NRCTs were also judged to be at moderate risk of bias for the relevant outcomes. Despite the limitations of this systematic review and the available literature, we provide the most comprehensive summary on the effect of shared-care postoperative management in non-cardiac surgical patients.
Conclusions
There is limited data evaluating the effects of shared-care management in non-cardiac surgical patients. The data that is available does not suggest an effect of shared-care postoperative management on mortality or length of stay in patients undergoing non-cardiac surgery. This systematic review identifies the need for well-designed, high-quality interventional studies to help guide clinical guidelines for non-cardiac surgery patients.
References
Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372: 139-44.
Bickler SW, Spiegel DA. Global surgery–defining a research agenda. Lancet 2008; 372: 90-2.
Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120: 564-78.
Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35: 2383-431.
Writing Committee for the Vision Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317: 1642-51.
Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MT, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307: 2295-304.
Devereaux PJ, Sessler DI. Cardiac complications and major noncardiac surgery. N Engl J Med 2016; 374: 1394-5.
Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370: 1504-13.
Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014; 28: e49-55.
Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33: 17-32.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.
Mendis N, Hamilton GM, McIsaac DI, et al. A systematic review of the impact of surgical special care units on patient outcomes and health care resource utilization. Anesth Analg 2019; 128: 533-42.
Stiefel M, Nolan K. A Guide to Measuring the Triple Aim: Population Health, Experience of Care, and Per Capita Cost. IHI Innovation Series white paper Cambridge, Massachusetts: Institute for Healthcare Improvement; 2012 .
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75: 40-6.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.
Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919.
Brockhaus AC, Grouven U, Bender R. Performance of the Peto odds ratio compared to the usual odds ratio estimator in the case of rare events. Biom J 2016; 58: 1428-44.
Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. Available from URL: http://handbook-5-1.cochrane.org/ (accessed March 2019).
Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol 2013; 66: 173-83.
Hempenius L, Slaets JP, van Asselt D, de Bock GH, Wiggers T, van Leeuwen BL. Outcomes of a geriatric liaison intervention to prevent the development of postoperative delirium in frail elderly cancer patients: report on a multicentre, randomized, controlled trial. PLoS One 2013; 8: e64834.
Hempenius L, Slaets JP, van Asselt D, de Bock TH, Wiggers T, van Leeuwen BL. Long term outcomes of a geriatric liaison intervention in frail elderly cancer patients. PLoS One 2016; 11: e0143364.
Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med 2004; 141: 28-38.
Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing 2007; 36: 190-6.
Chen W, Chang CC, Chiu HC, Shabbir A, Perng DS, Huang CK. Use of individual surgeon versus surgical team approach: surgical outcomes of laparoscopic Roux-en-Y gastric bypass in an Asian Medical Center. Surg Obes Relat Dis 2012; 8: 214-9.
McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994; 32: 40-66.
Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23: 1351-75.
Keus F, Wetterslev J, Gluud C, Gooszen HG, van Laarhoven CJ. Robustness assessments are needed to reduce bias in meta-analyses that include zero-event randomized trials. Am J Gastroenterol 2009; 104: 546-51.
Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007; 26: 53-77.
Acknowledgements
Manoj M. Lalu and Daniel I. McIsaac are supported by The Ottawa Hospital Anesthesia Alternate Funds Association and the Scholarship Protected Time Program, Department of Anesthesiology and Pain Medicine, uOttawa. The authors would like to thank Risa Shorr, MLIS, Learning Services, The Ottawa Hospital for assistance in generating the systematic search strategy.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Sasha Mazzarello and Manoj M. Lalu helped with study conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. Daniel I. McIsaac helped with study conception and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript. Josh Montroy helped with the acquisition, analysis, and interpretation of data. Dean A. Fergusson helped with study conception and design, analysis and interpretation of data, and critical revision of the manuscript. Dalal Yateem helped with acquisition of data and critical revision of the manuscript. P.J. Devereaux helped with study conception and design, analysis and interpretation of data, and critical revision of the manuscript.
Financial disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2019; 66: this issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mazzarello, S., McIsaac, D.I., Montroy, J. et al. Postoperative shared-care for patients undergoing non-cardiac surgery: a systematic review and meta-analysis. Can J Anesth/J Can Anesth 66, 1095–1105 (2019). https://doi.org/10.1007/s12630-019-01433-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01433-5